Citation: Van Ginneken T, “How COC Syringes are Shaping the Future of Injectable Drug Delivery”. ONdrugDelivery, Issue 178 (Oct 2025), pp 78–82.
Tom Van Ginneken explores the advantages of cyclo-olefin copolymer syringes, including where they outperform glass, which applications benefit most and why many common objections are outdated or debunked by current data, market adoption and technological advances.
“ORIGINALLY DEVELOPED IN THE EARLY 1990S AS A NEW CLASS OF AMORPHOUS OLEFIN-BASED POLYMERS, COC HAS SINCE EVOLVED INTO A LEADING MATERIAL PLATFORM FOR PARENTERAL PACKAGING, PARTICULARLY FOR PREFILLED SYRINGES AND OTHER DRUG DELIVERY SYSTEMS.”
In the ever-evolving landscape of injectable drug delivery, packaging and injection products play a crucial, yet often underappreciated, role in ensuring product safety, stability, usability and manufacturability. While borosilicate glass syringes have served the pharmaceutical industry well for multiple decades, new biologics, personalised therapies and complex drug formulations are demanding more and more from their delivery systems. Enter cyclo-olefin copolymer (COC) syringes – a modern, high-performance polymer-based alternative designed to meet the needs of both today’s and tomorrow’s drug products.
WHAT SETS COC APART AS A PACKAGING MATERIAL?
COC is a high-performance polymer that has gained widespread acceptance in pharmaceutical packaging due to its outstanding functional properties (Figure 1). Originally developed in the early 1990s as a new class of amorphous olefin-based polymers, COC has since evolved into a leading material platform for parenteral packaging, particularly for prefilled syringes and other drug delivery systems.
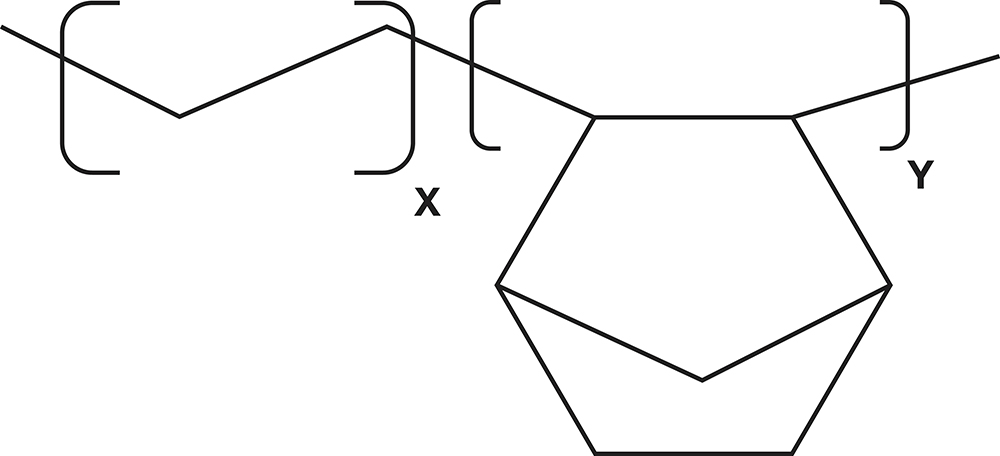
Figure 1: Chemical structure of cyclo-olefin copolymer.
COC is composed of cyclic and linear olefins, polymerised to form a rigid, non-reactive structure. It offers an exceptional combination of features that make it highly suitable for sensitive and high-value drug products:
- Superior Barrier Properties: COC exhibits low permeability to moisture and oxygen, offering effective protection for moisture-sensitive APIs and biologics, while supporting an extended shelf life.
- Chemical Inertness and Low Extractables: Unlike glass, COC contains no heavy metals or ions, eliminating the risk of ion leaching, thereby maintaining drug purity.
- No pH Shift: COC does not interact with aqueous drug solutions in a way that alters pH, maintaining formulation integrity over time – a critical factor for stability in biologics and vaccines.
- Excellent Optical Clarity: The material’s high transparency enables easy visual inspection of the filled product, meeting strict quality control requirements.
- Dimensional Stability and Precision: COC’s robust mechanical properties allow for high-precision injection moulding, ensuring consistent part geometry with tight tolerances and overall little product variation.
- Hydrophobic Surface: COC’s non-polar, hydrophobic nature reduces protein adsorption, preserving the structural integrity of protein-based drugs and minimising aggregation risks.
WHY CONSIDER COC SYRINGES?
When choosing a primary container for parenteral drug delivery, COC syringes offer more than just material advantages. Their comprehensive system design – inert material, cutting-edge cross-linked siliconisation, fully automated cleanroom production, high-quality rubber components and breakage-free performance – makes them an ideal solution for ensuring patient safety, drug stability, product integrity and manufacturing efficiency.
Cross-Linked Siliconisation
Cross-linked siliconisation is a next-level innovation that significantly reduces and immobilises the free silicone oil. This approach lowers sub-visible particle counts and minimises drug–silicone interactions, making COC syringes especially suitable for sensitive biologics and high-viscosity formulations.
Fully Automated, Human-Free Production
COC syringes are manufactured using a fully automated process, with no manual handling throughout production. This not only increases consistency and reduces variability but also eliminates the risk of human error and contamination.
Cleanroom Manufacturing for Ultra-Low Contamination
Every production step – from moulding to siliconisation and packaging – takes place within an ISO-classified cleanroom environment (Figure 2). This ensures the lowest possible bioburden and particulate contamination, aligning with the strictest regulatory and quality requirements.
Precision Moulding and Dimensional Accuracy
The injection-moulding process used for manufacturing COC syringes provides tight tolerances and excellent product reproducibility, which supports reliable functional performance as well as device integration in injection devices, infusion pumps and wearable drug delivery systems.

Figure 2: Production of COC syringes in a cleanroom – ensuring maximum product safety under controlled conditions.
ADVANTAGES OF COC SYRINGES OVER GLASS SYRINGES
Inert and Non-Reactive Container System
The borosilicate glass frequently used for pharmaceutical packaging is inorganic in nature, but multiple chemical elements are added to the silicon dioxide structure to make the glass more processable. These ions, such as sodium, can leach out of the glass structure and interact with the drug solution, potentially changing the pH of the solution. The hydrophilic, charged nature of the glass surface also promotes protein adsorption and aggregation, increasing the risk of denaturation and loss of drug efficacy. These interactions make borosilicate glass less ideal for modern biologic and high-value drug products.
COC, on the other hand, is free from ions and metal oxides, which eliminates the risk of drug interaction seen in some borosilicate glass compositions. Its non-polar, hydrophobic surface dramatically reduces the adsorption of proteins, peptides and other sensitive molecules, which is key for preserving drug stability, especially in sensitive biologic molecules and other advanced therapies.
Dimensional Precision and Consistency
Unlike glass syringes, which are subject to bigger tolerances inherent to the process of hot-forming the syringes out of glass tubes, COC syringes benefit from being manufactured using precision injection-moulding processes. This ensures tight dimensional tolerances, improving the consistency of product functionality and reducing mechanical failures in combination products such infusion pumps or injection devices.
Break Resistance and Patient Safety
COC syringes are shatter-proof, offering a clear safety advantage over glass, especially in home, paediatric or high-volume healthcare settings, reducing the risk of glass particulates, product recalls and potential patient harm. Furthermore, COC syringes are injection-moulded as a single piece, resulting in superior mechanical stability and structural integrity. Unlike glass syringes, where the Luer lock is added separately, the integrated Luer lock in COC syringes eliminates the risk of component separation or “needle pop-off” during injection or device use.
Low Drug Interaction with Cross-Linked Siliconisation
Cross-linked siliconisation is an advanced surface treatment used in COC syringes to ensure smooth gliding performance while minimising the drawbacks associated with traditional silicone oil lubrication. In this process, a silicone layer is chemically bonded and cross-linked to the inner surface of the syringe barrel, creating a stable, immobilised coating. Unlike conventional free silicone oil, which can migrate into the drug solution and lead to the formation of sub-visible particles, the cross-linked layer remains fixed in place, significantly reducing the risk of particle generation and drug–silicone interaction.
Greater Design Freedom
COC syringes offer greater design freedom due to being manufactured via injection moulding, which allows for highly precise and customisable geometries. Unlike glass, which has limitations in shaping and tolerance control, COC can be moulded into complex, integrated forms with tight dimensional accuracy. This enables the creation of features such as customised integrated Luer locks, optimised finger flanges or custom barrel designs tailored to specific drug delivery devices. Greater design flexibility supports better device integration, improved ergonomics and enhanced functionality for autoinjectors, wearable systems and specialised applications.
Safe and Reliable Performance in Cold Storage
In contrast with glass syringes, where leakage occurs after reaching a certain low temperature, COC syringes have been tested down to -180°C and still maintain their normal functionality and container closure integrity. The careful combination of COC (which has similar thermal expansion coefficient properties to halobutyl rubber), plunger rubber formulation, tight control of the syringe’s inner diameter and cross-linked silicone can ensure that even the lowest temperatures do not affect COC syringes.
WHEN IS A COC SYRINGE THE RIGHT CHOICE?
Neither glass syringe nor COC syringes are perfect. Both have their strengths and weaknesses, and both have their merits in the right conditions (Table 1). Therefore, understanding the requirements and the intended use is paramount when choosing the right syringe for a given application. The following are some common applications where COC syringes might be chosen over glass syringes:
Biologics and Biosimilars
COC’s inertness, low protein adsorption and particle control make it ideal for use with monoclonal antibodies, fusion proteins and complex formulations that would otherwise bind to or degrade in glass.
mRNA Therapies and Other Low-Temperature Storage Drugs
With messenger RNA (mRNA) vaccine storage often requiring cold-chain or cryogenic conditions, COC’s thermal stability and container closure integrity outperform glass. COC has been successfully used for deep-freeze applications.
Silicone-Sensitive Formulations
For silicone-sensitive biologics, the immobilised silicone in cross-linked siliconisation reduce risks of aggregation or immune responses compared with free silicone oil.
Hyaluronic Acid and Other Aesthetic Injections
COC syringes are ideal for aesthetic applications, such as dermal filler injections, where safety, precision and comfort are critical. Their integrated Luer lock prevents needle pop-off, ensuring secure administration and enhanced patient safety. The homogenous cross-linked siliconisation and precisely controlled inner syringe diameter enable smooth, consistent dosing, while ergonomic design features support user comfort and control during high-force injections, benefiting both patient and practitioner.
IV Drugs
Unlike glass syringes, where incompatibility with needleless intravenous (IV) access systems can cause leakage and, in the worst cases, cone breakage, COC syringes provide a safe and reliable injection solution for all IV injections.
| Glass Syringe | COC Syringes |
|
|
Table 1: The respective strength of glass versus COC syringes.
ADDRESSING COMMON OBJECTIONS TO POLYMER SYRINGES
Despite the growing adoption of COC syringes, some objections persist. Let us address them head-on:
“Polymers Are Too New”
COC is not new; it has been around for 30 years. Regulatory approvals for polymer syringes began more than 20 years ago, and they now support over 80 marketed drugs – including blockbusters – in over 90 regulated markets. This is not a leap into the unknown, it is a step towards proven innovation.
“Polymer Syringes Are Too Expensive Compared with Glass”
The assumption that polymers cost “several times more” than glass is outdated. Today, polymer syringe pricing is on par with standard biotech-grade glass syringes. When factoring in lower defect rates, fewer recalls, reduced breakage and improved device performance, the total cost of ownership can be significantly lower.
“Oxygen Permeation is a Major Issue in Polymers”
While it is true that COC is more permeable than glass, it is manageable. High- performance barrier labels, foil overwraps or secondary packaging can reduce oxygen ingress to acceptable levels, even for oxidation-sensitive molecules. These solutions are affordable and widely validated.
“Our Manufacturing Line is Built for Glass”
Many pharma companies have successfully adapted glass-compatible lines to handle COC syringes. With support from suppliers, often including technical assessments and change control documentation, polymer integration is frequently more feasible than assumed.
“The Supply Chain for Polymers is Too Risky”
This is not true anymore. The polymer syringe market now includes multiple qualified suppliers, including a second-source option using the same drug-contacting materials as the market leader. This allows for dual sourcing, reducing supply risk and enhancing flexibility.
“WHILE GLASS REMAINS SUITABLE FOR MANY CONVENTIONAL DRUGS, ITS LIMITATIONS ARE BEING INCREASINGLY EXPOSED BY NEW THERAPEUTIC FORMATS.”
THE BOTTOM LINE: FIT FOR THE FUTURE
Pharmaceutical development is advancing towards more complex, sensitive and patient-centric products. The delivery systems used must evolve in parallel. COC syringes offer:
- Superior safety through break resistance and low particulates
- Improved drug compatibility via inert surfaces and low adsorption
- Precision fit for high-tech devices
- Proven scalability and support for manufacturing and regulatory success
- Customisation to support safe and convenient patient-centric solutions.
Figure 3: WIM Ject® COC syringes in a tub: A break-resistant and cross-linked silicone alternative to conventional glass syringes.
While glass remains suitable for many conventional drugs, its limitations are being increasingly exposed by new therapeutic formats. COC syringes do not replace glass universally, but they offer a modern, performance-driven alternative where it matters most.
Wirthwein Medical helps pharmaceutical manufacturers find the ideal syringe for their application by either offering a standardised COC syringe – WIM Ject® (Figure 3) – or go one step further and offer individualised syringe solutions depending on the drug and specific needs of the application. Whether it is building up a second source for an already commercial COC syringe or finding the right syringe for a new application, Wirthwein Medical is able and happy to support.

