To Issue 134
Citation: Pager A, Despa M, “A Patient-Centric, Connected Injection Device for Decentralised Clinical Trials – the Human Factors Results Are In!” ONdrugDelivery, Issue 134 (Jun 2022), pp 66–70.
Aurélie Pager and Mircea Despa follow up on an article published in the December 2021 issue of ONdrugDelivery. BD has now completed a human factors study assessing the usability, acceptance and ease of use of its connected injection prototype for clinical trials.
“Following the positive results of human factors testing, the BD connected injection device prototype indicates itself as a promising technological approach to facilitate more efficient and cost-effective clinical trials through injection event data capture and reporting.”
The BD connected injection prototype by BD Medical Pharmaceutical Systems for use in decentralised and hybrid clinical trials addresses the needs of study sponsors, patients and clinical research organisations (CROs) for a safe, reliable, easy-to-use connected injection device that helps to overcome many of the challenges that arise during clinical trials. The basis of the BD connected prototype is an optional upgrade of the BD Ultrasafe Plus™ Passive Needle Guard.* The upgrade enables the device to automatically capture reliable, high-quality and time-stamped data from the injection event, with automatic data transmission to a cloud-based database of choice.
The challenges associated with clinical trials have been widely reported: growing trial costs and complexity,1 increased cycle time to database lock2 and non-adherence to protocol,3 followed by dropout and data quality issues due to labour-intensive data transcription and complex integration processes.4 Recruiting and retaining study participants also pose substantial problems5–7 due, in part, to travel and time burdens when participants are required to get to and from trial sites.
The covid-19 pandemic has both accentuated these challenges and accelerated movement towards change, in the form of decentralised clinical trials.8 Following the positive results of human factors testing, the BD connected injection device prototype indicates itself as a promising technological approach to facilitate more efficient and cost-effective clinical trials through injection event data capture and reporting.
BD is a world leader in injection devices, with billions of products produced each year. This connected injection device prototype is part of a larger focus at BD on patient-centric solutions. Its development aims to offer substantial benefits to the patient, healthcare and research communities alike. It represents the first step in bringing connectivity to a full range of BD drug delivery solutions.
THE TECHNICAL SOLUTION: MEETING PROJECT OBJECTIVES
BD set out to create a connected injection device with minimal perceivable differences compared with the standard, non-connected injection device. In other words, the connected aspect would neither interfere with the device’s functionality nor negatively impact its usability. With the BD Ultrasafe Plus™ Passive Needle Guard as the starting point, this meant integrating a wireless module (Bluetooth Low Energy) in such a way that it would go largely unnoticed by users – and in no way interfere or distract from the injection. To achieve this, the wireless module was integrated into the head of the injection prototype. This discreet module provides injection start and stop event data capture using a reliable, switch-based mechanism. Data emitted by the device, including the device unique identifier, is relayed to the cloud via an off-the-shelf smartphone device running a background app. The app functions as a “silent reporter”, requiring no actions by the user. The only user task beyond the injection itself is to ensure the phone is powered up with sufficient battery level and in the same room where injection takes place.
Previously reported test results9 confirmed that the connected version of the BD Ultrasafe Plus™ Passive Needle Guard does not modify device operation and closely aligns with the form factor and ergonomics of the base device (Figure 1).
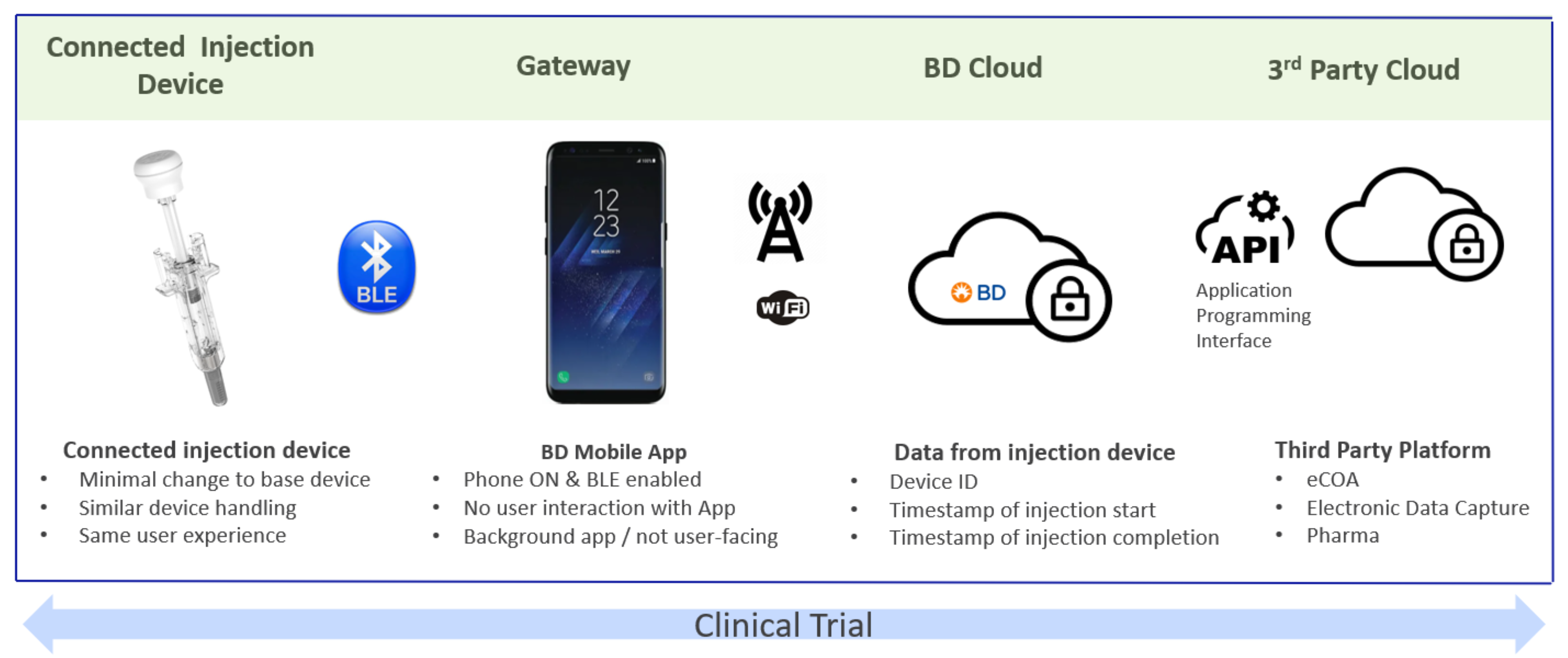
Figure 1: Contours of the BD connected solution. Informed by the research output, BD developed the solution architecture to match the needs of key stakeholders involved in clinical trials.
“The development of the connected BD Ultrasafe Plus™ Passive Needle Guard prototype was guided by quantitative and qualitative studies conducted among professionals in pharma, biotech, CROs and eCOA providers.”
BUILDING ON A USER-INFORMED DESIGN
The development of the connected BD Ultrasafe Plus™ Passive Needle Guard prototype was guided by quantitative and qualitative studies conducted among professionals in pharma, biotech, CROs and electronic clinical outcome assessment (eCOA) providers.3 In the context of those studies, presentation of the connected solution concept resulted in participants identifying multiple possible benefits. First and foremost, participants identified the timely detection of deviations from protocol as the most valuable potential benefit. Other perceived benefits included the elimination of manual data entry, avoidance of transcription errors and enhanced data quality, and automatic data transfer.
Asked what data such a device should capture in the context of clinical trials, the study participants identified device unique identifier, start time of the injection and the time of injection completion. Participants emphasised that the connected solution should not entail additional burdensome steps, that the form factor and function of the connected solution should closely align with that of the base device, and that the user should not be required to handle or interact with the phone at the time of injection. These studies laid the foundation for the design of the BD connected device prototype for clinical trials.
Once the prototype was developed, the device underwent a series of lab-based mechanical tests10 confirming that the connected solution was fully compatible with the base device. The evaluation confirmed complete dose delivery, successful triggering of the safety mechanism and equivalence in plunger activation force. The success of event capture and data transfer were also tested and found to be functioning as expected. The next step was to undertake a human factors study among user profiles corresponding to potential clinical study participants.
HUMAN FACTORS STUDY: DESIGN AND RESULTS
A human factors study was designed and executed to evaluate the functionality, usability, ease of use and acceptance of this connected version of the BD UltraSafe Plus™ Passive Needle Guard.11 The study included both on-site and home-based simulated subcutaneous injections. This proof-of-concept study provided an opportunity to simultaneously gather detailed observations and user feedback on the handling and use of the device and assess the automatically captured injection data. Study Design
The study took place during December 2021. The number of participants per user group was defined following human factors guidance,12 with participants recruited from three populations:
- Healthcare workers (HCW) (N = 7):
– Licensed and practising for at least two years, with experience with BD Ultrasafe Plus™ when possible. - Naïve patients (NVP) (N = 8):
– With chronic disease, when possible; if chronic disease, not receiving injectable treatment (oral treatment accepted); and not experienced with self-injection or with injections to others within the past 10 years. - Experienced patients (EXP) (N = 8):
– With chronic disease and without hand impairment; self-injection experience with prefilled syringes, syringe and vial or autoinjector; and experienced with BD Ultrasafe Plus™, when possible.
All participants undertook at least five simulated injections at the study centre, while participants in the two patient groups also performed two at-home injection simulations. Injection at the study centre took place over 45–90-minute sessions in a venue similar to a clinical setting and were video recorded. During those sessions, subcutaneous injections were simulated by injecting into a foam pad. At-home simulated injections took place with cut needle devices, by injecting into a cup or above a sink.
The data generated by the sessions were automatically collected into a BD database. In the case of an actual clinical trial, the data can readily transfer to a third-party platform, be it an eCOA tool, an electronic data capture system or directly to a dedicated pharma platform, via the use of application programming interfaces (APIs).
As shown in Table 1, all participants (n = 23) undertook at least three connected simulated injections on Day 0 at the study centre, with the HCW group also undertaking a fourth on Day 0. The two patient groups undertook at-home connected simulated injections on Days 3 and 6, with additional connected simulated injections at the study centre on Day 7.
| Day | Site | Who? | Total Planned Injections |
| D0 | Study Centre | HCW (n=7) NVP (n=8) EXP (n=8) |
84 |
| D3 | At Home | NVP (n=8) EXP (n=8) |
16 |
| D6 | At Home | NVP (n=8) EXP (n=8) |
16 |
| D7 | Study Centre | NVP (n=8) EXP (n=8) |
32 |
Table 1: Overview of human factors study design with planned connected simulated injections.
“With this first evaluation completed, the connected BD Ultrasafe Plus™ Passive Needle Guard prototype is ready for the next stage of development with the aim of being introduced in pilot clinical trials.”
Usability Confirmed
The usability of the BD connected prototype for clinical trials was confirmed in the study. For the initial in-clinic simulated injections, study participants were asked to do the simulations with no instructions or additional information available. Two incomplete simulated injections were observed during this first round of testing, committed by a single naïve participant who dismantled the device at cap removal, thus preventing the assessment of the following tasks. Use errors were seen mostly from the NVP and EXP groups related to cap removal and performance of the injection tasks. For all completed simulated injections, the entire dose was delivered and the safety guard was activated. After the initial injections, directions for use (DFUs) were made available to participants with no additional prompt or requirement to read them. For at-home simulations, specific instructions in addition to the DFUs were packaged along with the cut needle devices, the phones and the chargers.
Human Factors Study Results
Functionality results:
- Planned connected injections: 148
- Connected injections successfully completed: 146** (98.6%)
- Device failures: 0
- Cloud capture rate: 97%.***
Usability, ease of use and preference results:
- 23 participants (seven healthcare workers and 16 patients):
- 100% complete delivery****
- 100% safety activation****
- 93% of participants evaluated the connected prototype as easy or very easy to use*****
- 52% of the participants preferred the connected Ultrasafe prototype versus the standard reference product (30% of participants). The rest of the participants did not have a preference between the two products.
Key takeaways:
- BD Ultrasafe Plus™ Passive Needle Guard connected prototype performed as designed
- Excellent data capture rate, six-second median transmission time
- Zero new user risk identified
- No negative impact of connected plunger rod on users’ preference (Figure 2).
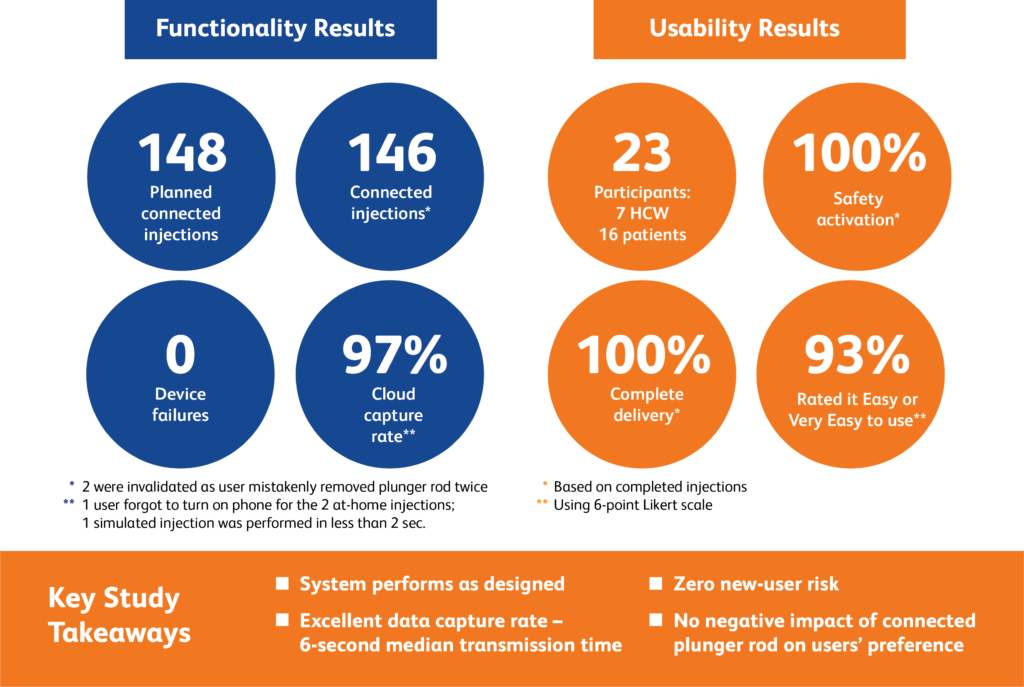
Figure 2: Human factors study at a glance.
GOING FORWARD
The human factors study discussed here indicates that the connected BD Ultrasafe Plus™ Passive Needle Guard prototype ensures reliable data transfer, enables near-real-time transmission of injection data and demonstrates similar usability results to the referenced standard product. With this first evaluation completed, the connected BD Ultrasafe Plus™ Passive Needle Guard prototype is ready for the next stage of development with the aim of being introduced in pilot clinical trials.
BD is presently seeking partners wishing to make this connected solution part of their future clinical trials for a new drug product, using the BD Ultrasafe Plus™ Passive Needle Guard. Timely detection of deviation from protocol, the elimination of transcription and accompanying errors, and fewer queries of drug intake data are some of the potential benefits the solution can offer.
* The connected injection prototype for the BD UltraSafe Plus™ is not a released product and is under development; some statements are forward-looking and are subject to a variety of risks and uncertainties.
** Of 148 planned connected injections, two were invalidated because one user mistakenly removed the plunger rod twice preventing injection completion.
*** The cloud capture rate was affected by three user behaviours (during the at-home simulations, one user did not turn the phone on, and one on-site injection was done so fast that the device did not register it).
**** Complete delivery and safety activation percentages based on completed injections.
***** Ease of use assessed using six-point Likert scale.
REFERENCES
- Getz KA, Campo RA, “Trial watch: Trends in clinical trial design complexity”. Nat Rev Drug Discov, 2017, Vol 16(5), p 307.
- “Industry Survey Reveals Clinical Data Management Delays Are Slowing Trial Completion”. PDF, Veeva, Sep 21, 2017.
- Delmas I, “Final Report: Value assessment of a subcutaneous connected device”. Internal Study, 2020, GLG Projects.
- Kondo H et al, “Evaluation of Data Errors and Monitoring Activities in a Trial in Japan Using a Risk-Based Approach Including Central Monitoring and Site Risk Assessment”. Ther Innov Regul Sci, 2021, Vol 55, pp 841–849.
- Chaudhari N et al, “Recruitment and retention of the participants in clinical trials: Challenges and solutions”, Perspect Clin Res, 2020, Vol 11(2), pp 64–69.
- Ross S et al, (1999), “Barriers to Participation in Randomised Controlled Trials: A Systematic Review”, J Clin Epidemiol, 1999, Vol 52(12), pp 1143–1156.
- Tudur Smith C et al, “The Trials Methodological Research Agenda: Results from a Priority Setting Exercise”. Trials, 2014, Vol 15, Article 32.
- “No place like home? Stepping up the decentralization of clinical trials”. McKinsey & Company, June 10, 2021.
- Beck R, Monchoix H, “Trials without tribulations: A patient-centric, connected injection device for decentralised and hybrid clinical trials”. ONdrugDelivery Magazine, Issue 128 (Dec 2021), pp 40–43.
- Internal BD lab report, 15R00038 OBWAN Post-Study Report, 2021.
- “Early Formative Human Factors Study of Connected Ultrasafe PLUS for Clinical Trials”. Internal study, 2021, Le Pont-de-Claix, France; Becton Dickinson.
- “Industry Guidance: Human Factors Guidance IEC TR62366-2:2016 Medical devices-Part 2: Guidance on the application of usability engineering to medical devices”. ISO, Apr 2016.

