To Issue 178
Citation: Guasti M, “Advancements in Intradermal Delivery: From Historic Techniques to Modern Innovations”. ONdrugDelivery, Issue 178 (Oct 2025), pp 50–54.
Michele Guasti explores the evolution of intradermal delivery methods, comparing historic methods of delivery, such as the Mantoux technique, with modern innovations, such as the Terumo Immucise™ intradermal injection system. The article goes on to discuss the expanding applications of intradermal delivery, including its potential for targeted lymph node delivery.
Intradermal (ID) delivery has long been a critical method in the medical and pharmaceutical fields, offering unique advantages for diagnosis, vaccination and therapeutic applications.
ID delivery involves administering substances directly into the dermis, the skin layer rich in immune cells. This method has been used for over a century, primarily for vaccinations and diagnostic tests. The dermis is extremely rich in various resident and recruited types of dendritic cells, which play a critical role in the human immune response by capturing antigens and presenting them to T cells. ID administration of vaccines can potentially result in quantitatively or qualitatively superior immune responses compared with intramuscular (IM) or subcutaneous (SC) injection.1
Terumo Corporation recently announced the commercial launch of its Immucise™ Intradermal Injection System, designed to deliver vaccines and other approved drugs to the dermal layer of the skin. This is a vertical puncture type ID injection device that is applied perpendicular to the surface of the skin in the deltoid region.2
This article aims to compare historic ID delivery methods with modern advancements and explore new applications, particularly in the context of targeted lymph node delivery.
“ID DELIVERY HAS A RICH HISTORY, WITH SEVERAL TECHNIQUES AND DEVICES DEVELOPED OVER THE YEARS, WITH THE AIM OF IMPROVING THE EFFICACY AND ACCURACY OF SUBSTANCE ADMINISTRATION INTO THE DERMIS.”
HISTORIC ID DELIVERY METHODS
ID delivery has a rich history, with several techniques and devices developed over the years, with the aim of improving the efficacy and accuracy of substance administration into the dermis.
The Mantoux technique, developed in the early twentieth century, is one of the most well-known methods for ID delivery. In the US, the Mantoux tuberculin skin test has been the standard method for detecting latent tuberculosis (TB) infection since the 1930s. To perform the ID injection, the needle is inserted slowly with the bevel against the patient’s skin at a 5°–15° angle. The needle bevel is advanced through the epidermis to a depth of approximately 3 mm. The injection will produce inadequate results if the needle angle is too deep or too shallow. The tuberculin solution is then slowly injected and a tense, pale wheal of 6–10 mm in diameter appears over the needle bevel.3
The Mantoux technique has several advantages, demonstrated by decades of clinical and epidemiological research in testing for TB in the US, where the technique has shown its simplicity (e.g. no laboratory equipment needed) and low cost.4 However, the accuracy and the consistency of an ID administration by the Mantoux technique with a standard syringe and needle mostly depend on the performance of the practitioner.5
Advancements in technology have led to the development of modern devices aiming to enhance the precision, ease of use and patient comfort in ID delivery.
THE IMMUCISE™ INTRADERMAL INJECTION SYSTEM: CONSIDERATIONS FOR PRACTITIONERS AND PATIENTS
Terumo’s Immucise™ Intradermal Injection System has been designed to deliver vaccines and other approved drugs to the dermal layer of the skin. The system is designed to be simple enough to handle easily and is expected to reduce risks of damaging tissues, such as blood vessels and peripheral nerves, due to its thin and short needle.5 In use, the system has advantages over traditional ID delivery methods.

Figure 1: The needle-end of the Terumo Immucise™ ID injection system, illustrating the outer diameter of 0.2 mm (33G) and length of 1.15 mm, which is designed to be retained in the dermal layer during delivery.2
First, the device is applied perpendicular to the surface of the skin in the deltoid region, supporting precise and consistent delivery.2 In a US FDA guidance-based human factors engineering study on the Immucise™ Intradermal Injection System, (“Immucise™ Intradermal Injection System Human Factors and Usability Engineering Study Report”), conducted by Terumo, a group of healthcare professionals (55 participants, including physicians, primary care nurses and pharmacists) were able to complete an injection using the Immucise™ Intradermal Injection System without use error on critical tasks, without receiving any prior instructions on the system. Furthermore, delivery was conducted without causing serious harm through accidental needlestick injury. This differs from the Mantoux technique, where the accuracy and the consistency of ID administration, by using a standard syringe and needle, mostly depends on the ability of the practitioner.5
The needle of the Immucise™ Intradermal Injection System is sized (i.e. outer diameter, bevel and length) so that the needle tip is retained in the dermal layer of the skin. The needle has an outside diameter of 0.2 mm (33G) and is 1.15 mm long, with a bevel length of 0.6 mm (Figure 1).2
The Immucise™ Intradermal Injection System is FDA cleared and indicated for ID injections of FDA-approved drugs. The system is to be used in the deltoid region for infants aged two months (excluding low birth weight and/or preterm birth) to adults.
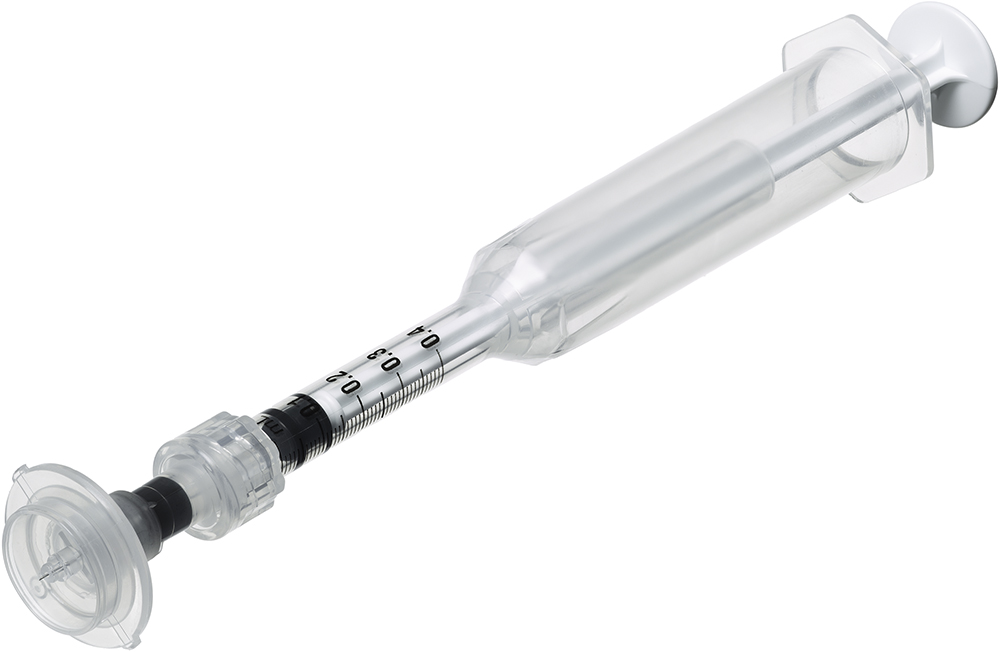
Figure 2: Immucise™ Intradermal Injection System composed of Immucise™ needle and Immucise™ syringe.
The device is also CE marked and is intended to be used for the ID injection of approved formulations (Figure 2).
ID VACCINATION
Driven by its innate ability to generate an enhanced immune response, dose sparing and the expansion of its use to deliver a wider variety of vaccines and other therapeutics, ID delivery is an increasingly popular route for vaccination.1
Enhanced Immune Response
The dermis is extremely rich in various resident and recruited types of dendritic cells, which play a critical role in the human immune response by capturing antigens and presenting them to T cells. ID administration of vaccines can potentially result in quantitatively or qualitatively superior immune responses compared with IM or SC injection.1
Dose Sparing
As the ID route enhances immune responses, smaller doses of a vaccine can achieve the same immunogenic effect as higher doses administered through other delivery routes. Numerous studies have demonstrated these dose-sparing effects for ID delivery of several vaccines, such as seasonal influenza and rabies. This is particularly valuable during vaccine shortages or in low-resource settings and allows for dose-sparing, making vaccines more accessible and cost-effective.1
Expansion of Use
ID delivery is already used for certain vaccines, such as the rabies and BCG (tuberculosis) vaccines.1 This established use provides a foundation for expanding ID delivery to other vaccines, using its potential for broader immunisation efforts.
CLINICAL TRIALS
In a paper titled, “Immunogenicity and safety of the new intradermal influenza vaccine in adults and elderly: A randomised Phase 1/2 clinical trial”, Ryo Arakane et al investigated the immunogenicity and safety of single, two-dose vaccination and three different dose strengths (6, 9 and 15 g HA per dose) of the influenza vaccine using the Immucise™ Intradermal Injection System and compared it with the standard SC vaccine (15 g HA per dose).5
The Immucise™ ID injection system showed a similar safety profile to that of SC administration. The seroprotection rate for strain B by ID administration of 15 µg of influenza vaccine was 69.4%, whereas by SC administration the rate was 48.0%. Administration of a 15 µg ID influenza vaccine with Immucise™ Intradermal Injection System showed a higher seroprotection rate compared with 15 µg SC dose in elderly subjects ≥ 65 years in Phase I/II trials trials (Figure 3).5
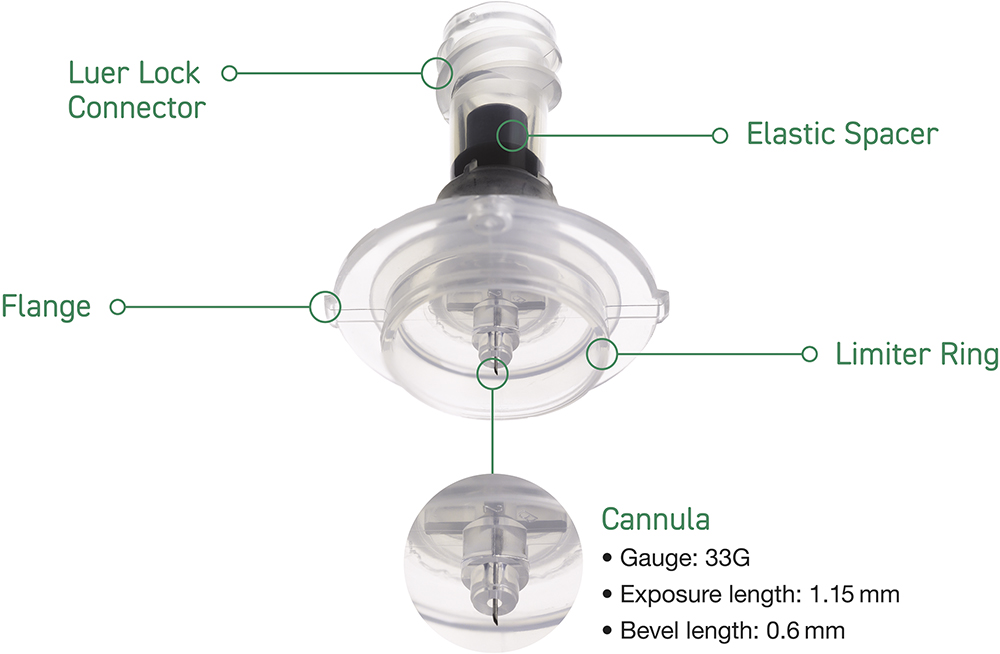
Figure 3: Immucise™ Intradermal Injection System.
Compared with the SC groups, both the single-dose ID group (until 21 days after administration) and two-dose ID group (until 42 days), showed higher geometric titers (GMT) for two type A strains.5 An additional study reported the GMTs of each strain at 90 and 180 days after vaccination in the elderly subjects.2 The ID group showed similar or better efficacy than the SC group until 180 days after vaccination in both the single- and two-dose groups. Notably, the GMTs in the B strain were significantly higher in the two-dose ID group than in the two-dose SC group in elderly subjects (p = 0.006 at day 90; p = 0.013 at day 180). These results show that ID administration may induce immunity for a longer period compared with the general administration route.2
“ID DELIVERY ALLOWS FOR LOCALISED ADMINISTRATION, WHICH CAN BE BENEFICIAL FOR TREATMENTS TARGETING SPECIFIC SKIN CONDITIONS OR LOCALISED IMMUNE RESPONSES.”
THERAPEUTICS
Beyond vaccines, ID delivery is being explored for the administration of biologics and other drugs.6 This method can improve the bioavailability and efficacy of these treatments. ID delivery allows for localised administration, which can be beneficial for treatments targeting specific skin conditions or localised immune responses.
POTENTIAL FOR LYMPH NODE DELIVERY
One of the most promising applications of ID delivery is its potential for targeted lymph node delivery, which may enhance immune responses and therapeutic outcomes.
ID delivery can target lymph nodes by using the rich network of lymphatic capillaries in the dermis.7 Lymphatic capillaries are 10–60 μm in diameter, around three-times the width of blood capillaries.8 They also have larger gaps between their endothelial cells compared with blood capillaries, which can expand to allow larger particles, such as proteins, lipids and even pathogens, to enter the lymphatic system.
Furthermore, lymphatic capillaries are designed to absorb interstitial fluid, which includes particles and molecules that have leaked out of blood capillaries. Unlike blood capillaries, lymphatic capillaries have an incomplete, discontinuous or absent basal lamina,8 and overlapping endothelial cells, which can act like one-way valves to permit the uptake of larger particles. Due to their unique structure, particles with sizes of 10–100 nm are preferentially taken up by the lymphatics.9
“STUDIES HAVE EXPLORED THE POTENTIAL OF ID DELIVERY FOR TARGETED LYMPH NODE THERAPIES, WITH PROMISING RESULTS IN CANCER TREATMENT.”
Further Promise for ID Delivery
For immunotherapies, delivering therapeutic agents directly to the lymph nodes can enhance their ability to stimulate an immune response against cancer cells or other pathogens, and studies have explored the potential of ID delivery for targeted lymph node therapies, with promising results in cancer treatment.
Furthermore, ID administration immediately and efficiently delivers antibodies to lymph nodes, whereas little drug is delivered by systemic administration.10
Drug delivery to lymph nodes – by changing the route of administration to ID – may improve drug efficacy, and ID administration of anti-PD-L1 antibodies has been found to significantly suppress tumour growth.10
Further research in this area is focused on developing methods to optimise the delivery of therapeutic agents to the lymph nodes, improving their efficacy and reducing side effects. Clinical trials are underway to evaluate the effectiveness of ID delivery for various therapeutic applications.
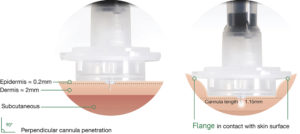
Figure 4: Immucise™ Intradermal Injection System (with dermal layer).
CONCLUSION
ID delivery has evolved significantly from its historic roots, with modern innovations, such as Terumo’s Immucise™ Intradermal Injection System, designed to offer enhanced precision and ease of use (Figure 4). Through broadening the application of this method to include a wider range of vaccines and therapeutic agents, ID delivery has the potential to revolutionise healthcare. Future research and development will continue to expand the possibilities of ID delivery, including its potential for targeted lymph node therapy.
Terumo Internal Reference: PM-09811
REFERENCES
- “Intradermal delivery of vaccines: A review of the literature and the potential for development for use in low- and middle-income countries”. PATH, Aug 2009.
- Shimizu S et al, “Performance and usability evaluation of novel intradermal injection device Immucise™ and reanalysis of intradermal administration trials of influenza vaccine for the elderly”. Vaccine, 2022, Vol 40(6), pp 873–879.
- “Mantoux Tuberculin Skin Test Facilitator Guide”. PDF, CDC, accessed Sep 2025.
- “Clinical Testing Guidance for Tuberculosis: Tuberculin Skin Test”. PDF, CDC, accessed Sep 2025.
- Arakane R et al, “Immunogenicity and safety of the new intradermal influenza vaccine in adults and elderly: A randomized phase 1/2 clinical trial”. Vaccine, 2015, Vol 33(46), pp 6340–6350.
- “New Technology Brings Simplicity & Scalability to Intradermal Drug Delivery”. Drug Development & Delivery, Mar 2018.
- Skobe M, Detmar M, “Structure, function, and molecular control of the skin lymphatic system”. J Investig Dermatol Symp Proc, 2000, Vol 5(1), pp 14–9.
- Lala PK, Nandi P, Majumder M, “Roles of prostaglandins in tumor-associated lymphangiogenesis with special reference to breast cancer”. Cancer Metastasis Rev, 2018, Vol 37(2–3), pp 369–384.
- Bagby TR et al, “Impact of molecular weight on lymphatic drainage of a biopolymer-based imaging agent”. Pharmaceutics, 2012, Vol 4(2), pp 276–295.
- Tanaka R et al, “Efficient drug delivery to lymph nodes by intradermal administration and enhancement of anti-tumor effects of immune checkpoint inhibitors”. Cancer Treat Res Commun, 2023, Vol 36, art 100740.

