To Issue 178
Citation: Sawyer B, Guss Tarazona E, Latham M, Bowen C, Sayed J, Field H, “Accelerating Time to Market for New Injection Devices Using a Novel Force-Modelling Method and Software”. ONdrugDelivery, Issue 178 (Oct 2025), pp 60–62.
Bradley Sawyer, Elena Guss Tarazona, Matthew Latham, Charlie Bowen, Dr Jay Sayed, and Harriet Field describe new software co-developed by Sanner Group and Pfizer to translate syringe test data into injection device performance, and to work backwards from device performance after shelf-life expiration to the maximum forces they can allow in a syringe test.
New injection devices are being asked to deliver larger volume injections, with higher viscosity formulations and increasing user expectations for device aesthetics, simplicity and quality. All injection devices risk performing inadequately when challenged with components, volumes and viscosities at the upper end of their tolerances. This risk of failure is present when developing devices for biosimilars and generics too.
As such, it is becoming ever more important to predict a device’s behaviour early in development, as well as to predict the effect of shelf-life ageing. However, with increased drug viscosity and volume comes more complex force requirements, and basic methods of predicting plunger forces are not sufficiently capable. The nightmare scenario is that an injection device is designed, taken through tooling, automation and design verification testing, and then fails its performance requirements at the end of its shelf life.
Sanner Group’s Design Centre of Excellence (Springboard) has developed new software that allows the translation of basic syringe test data into injection device performance. The software also enables engineers to work backwards from device performance after shelf life to the maximum forces they can allow in a syringe test. This can be done early in development to avoid failures late in the programme.
WHY USE BREAK-LOOSE EXTRUSION FORCE DATA?
Break-loose and extrusion force (BLEF) tests are a common way to characterise prefilled syringes with plungers. In a BLEF test, a tensometer drives a plunger at constant speed and measures the (varying) force as the plunger moves down the syringe.
The force-distance chart created in a BLEF test provides useful information – the initial break-loose force of the stationary plunger, the extrusion force (which comprises the dynamic plunger-syringe friction and the drug formulation’s back-pressure) and a small dip in force indicative of an air bubble expelled after drug delivery (Figure 1).
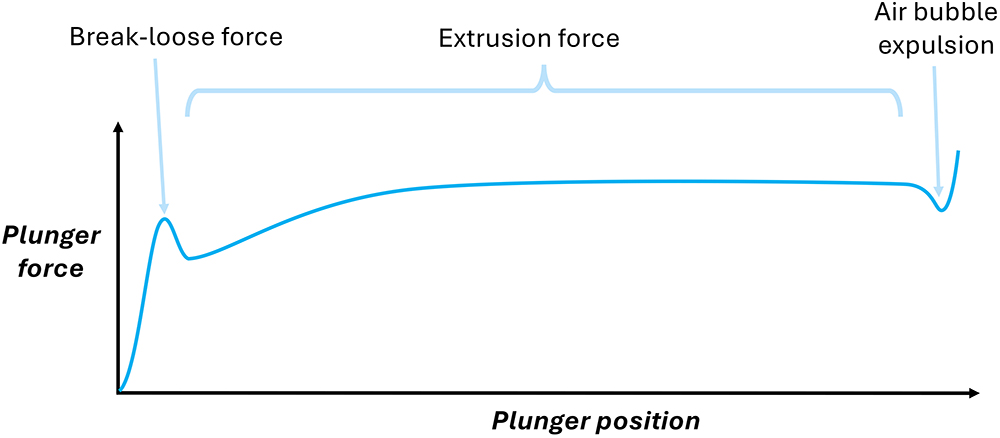
Figure 1: The force-distance chart for a BLEF test.
ISO 11040-8 mandates that such tests use the complete, final system “as intended for use” and that the designer considers how forces vary with ageing and environmental factors. BLEF testing is valuable for characterising the relationships between forces and plunger speed, syringe or needle dimensions, and fluid properties. However, it cannot be used to accurately predict injection times for a device because it does not reflect dynamic injection behaviour. Therefore, designers need a tool that can use common BLEF test data to provide accurate predictions for injection device performance.
To address this, Springboard has co-developed advanced modelling software with Pfizer’s Devices Centre of Excellence. This software enables device developers to easily forecast injection times, anticipate variation due to ageing and set meaningful specifications early in the development process. The software can bridge the gap between constant-speed BLEF data and dynamic injection device performance without the need for guesswork, reducing the number of slow and costly prototype testing cycles and rework (Figure 2).

Figure 2: Steps taken to generate BLEF predictive data.
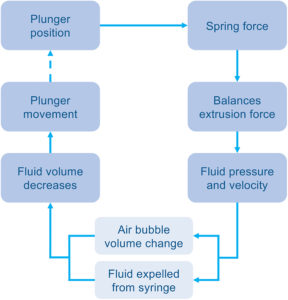
Figure 3: A time-stepped approach to break down an injection.
A NEW MODEL AND SOFTWARE FOR DEVICE PERFORMANCE
Software users simply upload their own BLEF data into the software and, in a few steps, can generate:
- A predicted distribution of injection times for their specified device
- Maximum extrusion force requirement predictions
- A report for their design history file.
The model behind the software uses a time-stepped approach to break down an injection into discrete, calculable steps (Figure 3). Each loop begins with a known plunger position, which relates to the device’s spring length. From this, the spring force is known and is assumed to be approximately equal to the current extrusion force.
Using relationships from the user’s BLEF data for the modelled syringe, the extrusion force is used to work back to the plunger speed and internal syringe pressure. By calculating the Hagen-Poiseuille equation across the needle, the pressure is related to the velocity of fluid ejected.
The plunger movement can then be calculated due to the reduction in injection volume, while also accounting for any changes in any air bubble volume due to pressure changes – this step is important to capture, but is complex and challenging to address through other predictive methods. The loop is repeated until the full volume has been delivered, with the total time elapsed giving the device injection time.
This software uses the Monte Carlo method to sample the random variation of all input parameters, which include:
- User-specified tolerances on input parameters
- The variance of real BLEF data.
“VARIOUS FACTORS LEAD TO DEVICE PERFORMANCE DEGRADATION OVER TIME, MOST NOTABLY DESILICONISATION
OF THE SYRINGE, WHICH LEADS TO INCREASED PLUNGER-SYRINGE FRICTION.”
COPING WITH DEVICE AGEING
Various factors lead to device performance degradation over time, most notably desiliconisation of the syringe, which leads to increased plunger-syringe friction. After shelf-life expiration, device performance is more likely to fail requirements such as injection time than on the day the device was manufactured. This is bad news for development projects because issues might not be discovered until years into a programme.
Therefore, Sanner Group and Pfizer used newly siliconised and fully desiliconised syringes as best and worst cases for plunger-syringe friction and used desiliconisation as a surrogate for ageing. In practice, while it is safe to say that syringes effectively move away from newly siliconised performance as they age, most will never get near to fully desiliconised, depending on their ageing properties.
“THE SOFTWARE HAS BEEN EXTENDED TO ESTABLISH
AGEING TRENDS.”
The software has been extended to establish ageing trends using a small number of single-speed BLEF tests on aged syringes. This effect is modelled by increasing theoretical BLEFs until devices stall or breach injection time limits. This results in establishing the worst case syringes that can be allowed. Then, by back-calculating from end-of-shelf life performance to initial conditions, the method can define a maximum allowable BLEF result at time zero. This allows engineers to specify the maximum forces allowed on a BLEF test at time zero where they can still be confident that the injection device would perform after shelf-life expiration.
PRECISION ENGINEERED – SEAMLESSLEY DELIVERED
As user expectations for device aesthetics, simplicity and quality continue to rise, the pressure on the medical device industry intensifies. Sanner empowers pharmaceutical and healthcare companies to meet these demands by uniting world-class design expertise, global manufacturing capabilities, regulatory know-how and operational excellence. From next-generation injection systems and drug delivery devices to innovative diagnostics and connected health solutions, Sanner delivers a comprehensive, single-source approach that accelerates time to market. Built on a foundation of quality, trust and shared purpose, Sanner stands ready to shape the future of medical device development in close partnership with its customers.
“DEVICE DEVELOPERS CAN USE THE NEW MODEL DESCRIBED HERE TO CHANGE THE PHYSICAL BUILD-AND-TEST LOOP INTO A MATHEMATICAL MODEL, WHICH SPECIFIES ACCEPTABLE FORCE LIMITS FOR EASY BLEF TESTS EARLY IN THE PROGRAMME.”
SUMMARY
If injection time performance is not properly predicted (including predictions for after shelf life), a device programme risks substantial cost and time overruns. The cost and time come from having to implement time-consuming and expensive component modifications and reverification testing.
Instead, device developers can use the new model described here to change the physical build-and-test loop into a mathematical model, which specifies acceptable force limits for easy BLEF tests early in the programme. These predictive tools will become increasingly essential for efficient and reliable device development as drug delivery systems evolve to accommodate more demanding formulations. The modelling software offers a practical solution by translating BLEF test data into more accurate forecasts of injection performance over a device’s lifespan.

