To Issue 142
Citation: Morel B, Corrons-Bouis, “An Intuitive All-In-One Autoinjector: Embedded Mixing & Injection Technologies to Simplify Day-To-Day Life”. ONdrugDelivery, Issue 142 (Feb 2023), pp 106–110.
Benjamin Morel and Gladys Corrons-Bouis, discuss the challenges faced by the injectable drug delivery industry and present EVEON’s solution – the INTUITY® Ject MX platform.
“Improving the patient experience for chronic injectable treatments
is now a critical challenge to address.”
Over the past few years, the pharmaceutical industry has faced a rapid shift in the preferred delivery route for its products, with the injectable route becoming increasingly prevalent in the development pipeline. According to the US FDA, 40% of new drugs approved are injectable products, with a great proportion of those targeting subcutaneous and intramuscular routes. In 2022, seven out of the top ten selling drugs were injectables, compared with one in 2003 and five in 2010. Consequently, the pharmaceutical industry has begun adopting new strategies and putting the patient first to improve treatment adherence and boost product sales. It now takes more than a promising molecule and a validated therapeutic target to gain traction in the market.
These trends come in tandem with a global increase in the incidence of chronic diseases, which has become one of the greatest global health threats of the 21st century. Worldwide, they caused 42 million deaths in 2019. Chronic diseases require chronic treatments, and important follow-up work by healthcare workers. As such, improving the patient experience for chronic injectable treatments is now a critical challenge to address.
CHALLENGES FOR THE INJECTABLES INDUSTRY
New Drugs in the Pipeline
The injectable drug delivery market is forecast to experience double-digit growth between 10% and 15%.1 It is a complex industry involving multiple interconnected partners, each of them able to solve or create challenges for the others. These players include drug developers, primary packaging companies and device companies. One of the key drivers impacting all of them is the aforementioned paradigm shift in the drug development pipeline that is shaping the future needs of the industry.
Biologics are having a major impact on market trends – they represent over double the value of the small molecule market and will continue to strongly support the global injectables market.2–4 However, a key challenge associated with biologics is their formulation and storage, meaning that about one-third of the market will come to involve lyophilisedproducts.1 For example, highly concentrated antibody formulations are often designed as lyophilised product, as liquid formulations frequently fail to pass stability tests.5
Due to the need for a diluent and a reconstitution step, lyophilised products present additional complexity prior to administration. Furthermore, reconstituting highly concentrated formulations can take several minutes due to the physical properties of the lyophilised cake.6 Therefore, it is necessary to find appropriate devices that facilitate easy reconstitution and injection of viscous lyophilised drug products.
New long-acting injectables (LAIs), or “depot-injection formulations”, are currently of high interest due to their potential in increasing patient adherence to treatments. LAIs have been used in the field of antipsychotic, antiviral and addiction treatments. Over the past few decades, LAI formulations have proven their worth as safe and effective products and are now true game changers in the field of controlled-release delivery. However, ensuring their stability and homogeneity over time remains a great challenge. Most LAI formulations are water-insoluble, which requires drug delivery solutions that can handle their viscosity and ensure that they are properly mixed in order to avoid clog formation during injection.
“The pharmaceutical industry currently faces a major unmet need – offering to patients an intuitive, easy-to-use autoinjector that enables automatic reconstitution, mixing and injection.”
Devices and Product Lifecycle Management Strategies
Product lifecycle management involves finding ways to maximise the value of the product and retain market share as patent expiry or market exclusivity rights approach expiration. Offering a new device solution that will enhance the patient experience is a key strategy for lifecycle management, which is currently gaining interest in the pharmaceutical industry.
From a device perspective, two key drivers can be outlined to explain future changes related to improving the patient experience within the injectables industry:
- The switch from hospital settings to at-home treatment is a key trend that received a boost from the covid-19 pandemic.7 Accordingly, there is a need to develop solutions that will fit with patients’ daily lives. In so doing, the medical device can become a key element for treatment adherence.
- The second important driver is digitalisation. Digital devices can provide injection guidance, dose confirmation and reminders, among other benefits. Additionally, digitalised devices offer an enhanced way to monitor patient adherence and maintain the link between patients and healthcare professionals.
INTUITY® JECT MX – SIMPLIFING PATIENTS’ DAILY LIVES

Figure 1: EVEON’s INTUITY® Ject MX platform. (Learn more by watching the video on EVEON’s YouTube channel, here.)
The pharmaceutical industry currently faces a major unmet need – offering to patients an intuitive, easy-to-use autoinjector that enables automatic mixing, dosing and injection. As of today, there is no state-of-the-art solution that is able to provide a full answer to this unmet need.
EVEON’s core expertise is focused on drug preparation, including precise drug mixing and dosing. As such, the company’s INTUITY® Mix platform has been designed to overcome the challenges presented by difficult-to-mix drugs, including:
• Liquid-to-liquid mixing: pooling of multiple doses, emulsion preparation, dilution.
• Liquid-to-solid mixing: lyophilised drug reconstitution, suspension preparation, powder dispersion.
To achieve this, EVEON developed a range of patented micropumps, dedicated to complex drug mixing. Recent developments regarding the company’s micropump technology were discussed in a previous ONdrugDelivery article,8 showcasing its strong mixing properties – even in its silicon-free version – to better comply with the constraints associated with delivering biologics. EVEON’s latest optimisation enables the preservation of mechanical properties while avoiding clog formation and particle aggregation due to the presence of silicon oil.
EVEON’s micropump is perfectly suited to handle high pressure (up to 10 bar), which enables strong mixing properties for lyophilised cake dissolution and powder dispersion in a highly viscous oil phase. However, while developing its expertise in drug mixing, EVEON found that new challenges arose regarding the need for an all-in-one autoinjector capable of full-automatic drug mixing, dosing and drug injection.
EVEON has developed INTUITY® Ject MX, which is shown here in Figure 1, with more information available in the video on EVEON’s YouTube channel. INTUITY® Ject MX is an all-in-one autoinjector platform integrating the company’s core mixing technologies. It is compatible with a broad range of standard primary containers, including vials, cartridges and syringes. Developments have been made to mix from two or more containers and to make the platform adaptable to multiple combinations of standard primary packaging types and sizes. Automatic mixing from cartridge to cartridge, vial to cartridge or syringe to cartridge are new possible options, opening new opportunities and providing flexibility to the industry.
Figure 2 provides a snapshot of the range of viscosities INTUITY® Ject MX is capable of handling, as well as data on its mixing and injection performance. Its key technical benefits include the reduction of preparation time, improvement of homogeneity and stability after mixing, and a reduction in clog formation compared with manual extemporaneous drug preparation. Figure 2 also shows performance data for suspension preparation, with stability time increased by two for oil-phase suspensions.
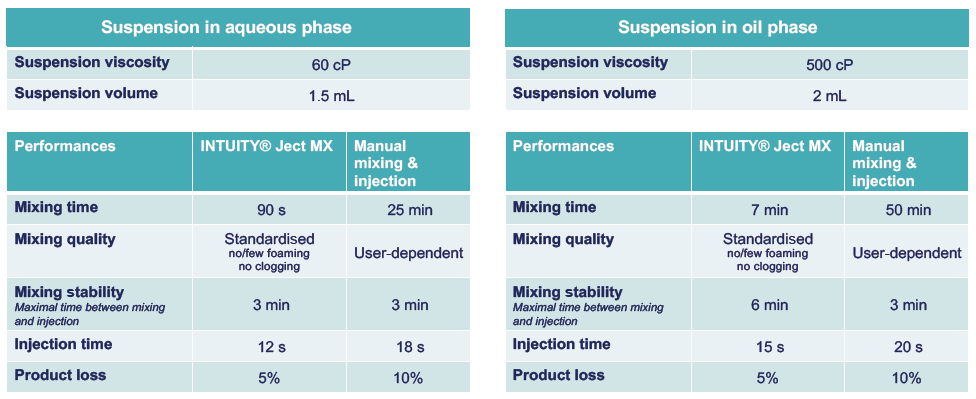
Figure 2: INTUITY® Ject MX performances regarding mixing and injection of LAIs for different viscosities and solvent type.
“The INTUITY® Ject MX platform opens new opportunities for the pharmaceutical industry, offering a fully automatic autoinjector that enables drug mixing, dosing and injection in the same handheld device.”
The INTUITY® Ject MX autoinjector platform relies on two side-by-side standard primary containers (vials or cartridges) and a custom fluidic cassette dedicated to receiving those two containers upon device activation. Many possibilities are available, from fully disposable devices with embedded drug containers to reusable devices with disposable fluidic cassettes and drug containers. The platform’s modularity enables it to be adapted for various patient needs and also presents a possible answer to the need for increasing the sustainability of medical devices. Indeed, the covid-19 pandemic exposed several drawbacks of disposable medical devices. Finding alternatives to limit medical waste by using a two-part device with a reusable injector and single-use fluidic cassette could be a solution for improving sustainability in future years.
In the platform’s fully automatic configuration, a single user action initiates the electronic activation and fluidic connection between the containers and the fluidic cassette. Then the device automatically starts mixing the powdered drug and the diluent and informs the patient when it is ready for injection. A motorised micropump with embedded software controls the number of back-and-forth movements, enabling perfect drug mixing. Furthermore, the injection can be triggered and controlled by the device to avoid unsuitable injections.
Studies have demonstrated that INTUITY® Ject MX enables both highly precise final concentrations after mixing and delivered dose volumes (Figure 3). Additionally, the fluidic cassette is fully customisable according to the primary drug containers used and factors such as the drug sensitivity to shear stress, homogeneity, preparation time and dead volume reduction.
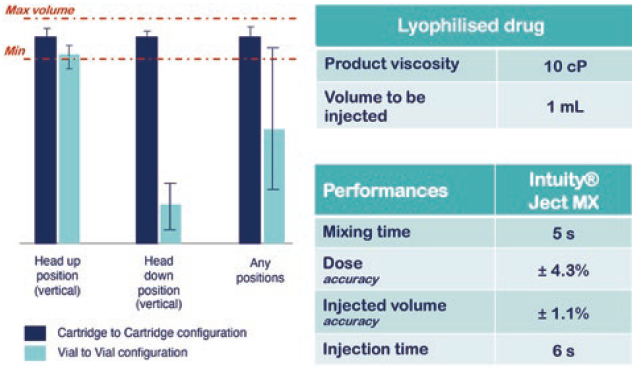
Figure 3: Use case of INTUITY® Ject MX for Lyo reconstitution.
Figure 4: EVEON’s digital companion for INTUITY® Ject MX.
INTUITY® Ject MX performance data demonstrate that the platform is able to mix and deliver a drug product regardless of orientation when using a two-cartridge configuration with no impact from the sticking effect of the cartridge stoppers. This enables new opportunities for use in emergency situations or for specific populations, such as children, disabled or the elderly, with fully automatic delivery from a single user action (Figure 3).
Additional key benefits are a drastic reduction in the number of steps required during product preparation, dosing and injection while also ensuring patient safety and standardised drug administration. INTUITY® Ject MX reduces the number of steps in a standard use scenario from about 20 steps to 5–8 steps, depending on the global level of automation required for the final application.
Finally, digital functionality has also been developed, enabling EVEON to offer a digital companion alongside the device (Figure 4). The companion provides dose-mixing monitoring, injection monitoring, injection reminders and an injection timer.
CONCLUSION
The INTUITY® Ject MX platform opens new opportunities for the pharmaceutical industry, offering a fully automatic autoinjector that enables drug mixing, dosing and injection in the same handheld device. INTUITY® Ject MX has both standard essential features and nice-to-have functionality that can be fine-tuned to each drug.
Figure 5 showcases the key benefits for both the pharmaceutical industry and patients. Positioned as an expert in drug preparation and having the right set of combined expertise in fluid physics, mechatronics and software, EVEON is the partner of choice for developing an advanced custom drug delivery device.

Figure 5: Key benefits of INTUITY® Ject MX.
Learn more about INTUITY® Ject MX by watching the video on EVEON’s YouTube channel, here.
REFERENCES
- “Injectable Drug Delivery Market By Devices Type (Convention Injectables, Pre-filled syringes, Auto-Injectors, Pen-Injectables, Wearable), Products (Freeze Dried Products, Injectable Sterile Products), Applications (Hormonal Disorders, Auto-Immune Diseases, Oncology, Orphan/Rare Diseases, Others), End-User (Hospitals, Home Care Settings, Others), By Geography, Size, Global Report, Forecast, 2021-2030”. Research Report, Strategic Market Research, Mar 2022.
- “Global biologics market is projected to grow at a CAGR of 8.82% By 2032″. Press Release, Visiongain, Aug 9, 2022.
- “Global Small Molecule Injectable Drugs Market Insights Forecasts to 2030”. Research Report, Spherical Insights, Oct 2022.
- “Injectable Drugs Market – Growth, Trends, COVID-19 Impact, and Forecasts (2023–2028)”. Research Report, Mordor Intelligence Report, Nov 2022
- Jiskoot W et al, “Ongoing Challenges to Develop High Concentration Monoclonal Antibody-based Formulations for Subcutaneous Administration: Quo Vadis?”. J Pharm Sci, 2022, Vol 111(4), pp 861–867.
- Kulkarni SS et al, “Key factors governing the reconstitution time of high concentration lyophilized protein formulations”. Eur J Pharm Biopharm, 2021, Vol 165, pp 361–373.
- Martin T, “The shift to home care: Another new normal resulting from Covid-19”. MedCityNews, Mar 10, 2021.
- Bouillet A, Girois L, Weidenhaupt M, “Enhancing Protein Preservation During Biological Drug Preparation & Delivery”. ONdrugDelivery, Issue 113 (Oct 2020), pp 46–48.

