Citation: Ibisate I, Garcia Nograro A, Incierte I, “Custom Metal Solutions for Emerging Challenges in Autoinjector Systems”. ONdrugDelivery, Issue 178 (Oct 2025), pp 72–76.
Iker Ibisate, Álvaro García Nograro and Irakusne Incierte address the complex manufacturing challenges posed by increasingly sophisticated autoinjector devices and introduce RPK Medical’s approach as a technical partner in overcoming these challenges through the tailored design and manufacture of functional metal components, such as precision springs and stamped parts.
The autoinjector device market is experiencing rapid growth, driven by the increasing demand for home-based treatments, biologic therapies and greater patient autonomy. These increasingly sophisticated devices pose complex engineering challenges related to miniaturisation, the delivery of highly viscous drugs, precise flow control, safe skin contact and digital connectivity. RPK Medical’s solutions not only address these challenges but also significantly contribute to the clinical and commercial success of next-generation drug delivery devices, positioning the company as a key player in the industry.
The autoinjector market is expanding at a double-digit rate, primarily driven by the growth of biologic therapies and the increasing preference for self-administration outside hospital settings. These devices must be safe, user-friendly and compatible with increasingly complex drug formulations. Simultaneously, they must meet new demands surrounding portability, sustainability, connectivity and regulatory compliance.
“WITH ITS EXTENSIVE EXPERTISE IN DESIGNING AND MANUFACTURING METALLIC COMPONENTS FOR MEDICAL DEVICES, RPK MEDICAL PROVIDES TAILORED SOLUTIONS THAT EFFECTIVELY AND RELIABLY ADDRESS THESE CHALLENGES, INSTILLING CONFIDENCE IN THE COMPANY’S ABILITY TO MEET CLIENT NEEDS.”
In this context, precision mechanical engineering plays a pivotal role. Components such as compression springs, stamped parts or metal contacts are no longer mere structural aids; they perform critical functions, including needle deployment, plunger actuation, flow control and dose delivery. With its extensive expertise in designing and manufacturing metallic components for medical devices, RPK Medical provides tailored solutions that effectively and reliably address these challenges, instilling confidence in the company’s ability to meet client needs.
MINIATURISATION: FUNCTIONALITY IN COMPACT FORMATS
The trend towards more ergonomic, portable and sustainable autoinjectors requires a significant reduction in component size and volume, without compromising critical functions, such as activation, needle retraction, sensor integration or visual/acoustic indicators, posing a significant engineering challenge. Miniaturisation addresses the clinical need for discreet, user-friendly and easily disposable devices, enhancing patient satisfaction and overall experience (Figure 1).
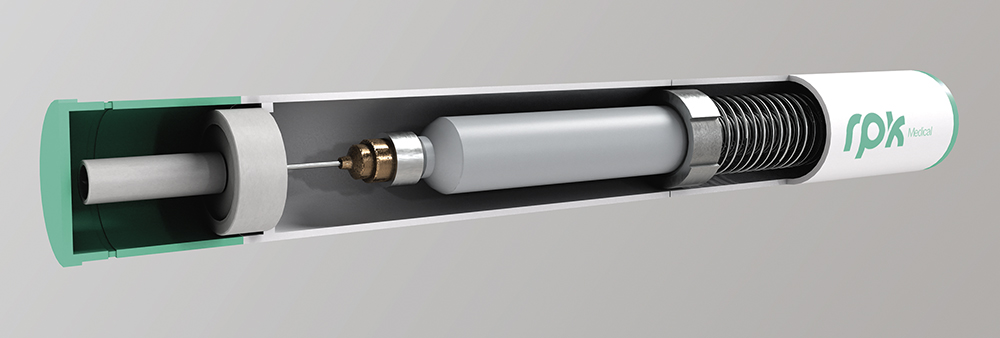
Figure 1: Miniaturised autoinjector with precision springs and metal components.
However, reduced space makes the inclusion of essential safety mechanisms – such as needle locks or post-use protection features – difficult, demanding optimal use of every single millimetre of internal design. This need for precision requires components to be manufactured to extremely tight tolerances to ensure precise assembly and reliable operation in compact devices. Some of the technical challenges include:
- Space limitations for critical mechanisms
- Strict dimensional tolerances to prevent assembly errors
- Co-existence with electronics, batteries or actuators in constrained areas.
RPK Medical’s solutions include:
- Micro-springs optimised for compact geometries using finite element analysis
- Multifunctional stamped parts combining structural and mechanical roles
- Rapid prototyping and co-engineering for agile design iteration.
DELIVERY OF HIGHLY VISCOUS DRUGS
Modern biologic therapies – such as monoclonal antibodies and advanced treatments for immune, oncologic or neurodegenerative conditions – are often highly viscous, making them difficult to administer with conventional autoinjectors. They require greater plunger force and precise speed control to prevent pain, dosing errors or incomplete injections. These concentrated formulations offer more capable, longer-acting treatments, but they require injection solutions adapted to their rheological properties (Figure 2).
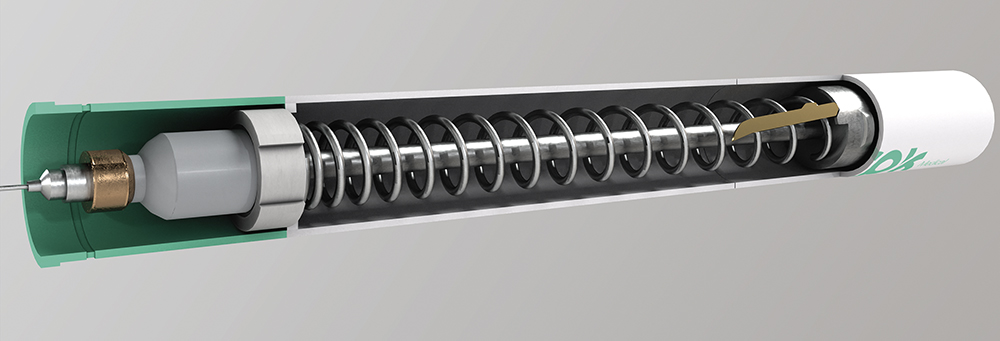
Figure 2: Flow control system for high-viscosity drugs.
Delivering highly viscous fluids requires overcoming greater resistance and carefully modulating the flow to avoid discomfort or underdosing – especially during slow subcutaneous injections that last 30 seconds or more. It is crucial to prevent both flow drops and pressure spikes, as these can compromise patient experience or treatment efficacy. The core challenge is designing mechanisms capable of delivering these formulations reliably, safely and almost imperceptibly to the user. Some of the technical challenges include:
- High plunger resistance
- Risk of interrupted or partial dosing
- Need to maintain comfort and pressure control throughout administration.
RPK Medical’s solutions include:
- High-force springs in 17-7PH stainless steel or other specialised alloys
- Multistage mechanisms (e.g. concentric or telescopic springs) to modulate energy
- Rheological simulation and testing to match component behaviour to drug profiles.
FLOW CONTROL: STABILITY AND THERAPEUTIC EFFECTIVENESS
Maintaining a consistent injection flow rate is crucial for both therapeutic efficacy and patient comfort, as abrupt flow variations can lead to pain, bruising, poor absorption or subtherapeutic dosing. Unstable flow profiles may affect both the effectiveness of the drug and user acceptance of the device, eroding trust in treatment. This requirement becomes even more critical for smart autoinjectors and wearable systems (on-body delivery systems), which must deliver medication predictably over set intervals – from seconds to minutes – in accordance with clinical guidelines.
“PATIENT COMFORT DEPENDS ON SMOOTH, UNINTERRUPTED DELIVERY, WHILE REGULATORY STANDARDS REQUIRE CONSISTENT FLOW PERFORMANCE ACROSS ALL DEVICES AND USES, PRESENTING MAJOR DESIGN, VALIDATION AND QUALITY CONTROL CHALLENGES.”
Patient comfort depends on smooth, uninterrupted delivery, while regulatory standards require consistent flow performance across all devices and uses, presenting major design, validation and quality control challenges. Some of the technical challenges include:
- Internal pressure variability due to changes in viscosity or temperature
- Limited space for active controllers
- Regulatory demands for flow reproducibility.
RPK Medical’s solutions include:
- Constant-force springs for stable plunger actuation
- Calibrated stamped parts used as flow restrictors, passive valves, or energy dissipators
- Mechanical alternatives to electronic pumps or actuators.

Figure 3: Skin-contact mechanism in an autoinjector.
RELIABLE SKIN CONTACT
The interaction between the autoinjector and the skin is a critical clinical and technical factor. Poor contact may result in failed administration, surface injury or loss of user confidence. Devices must adapt to varying body morphologies, applied pressure levels and application zones (such as the abdomen, thigh and arm), all with different curvatures and mobility, while preventing slippage or accidental triggering. The challenge intensifies in unsupervised home settings or among vulnerable populations (e.g. the elderly or those with limited mobility), where application errors may have a serious impact on health. Therefore, the physical interface must be designed with maximum ergonomics and safety in mind, ensuring the device only activates when optimal skin contact is achieved – accounting for skin thickness, hair, sweat and irregular surfaces (Figure 3). Some of the technical challenges include:
- Anatomical variability and inconsistent manual pressure
- Risk of incomplete or accidental activation
- Need for clear mechanical feedback to the user.
RPK Medical’s solutions include:
- Calibrated springs ensure consistent skin contact pressure
- Sensor-stamped parts that only trigger activation under optimal conditions
- Passive fixation systems or mechanical suction cups for wearable applications.
“THE DIGITAL EVOLUTION OF NEXT-GENERATION AUTOINJECTORS – FEATURING SENSORS, DATA LOGGING AND WIRELESS CONNECTIVITY – ENABLES USAGE TRACKING, PERSONALISED DOSING AND APP INTEGRATION, ALIGNING WITH TRENDS IN TELEMEDICINE AND REMOTE MONITORING.”
CONNECTIVITY AND DIGITAL INTEGRATION
The digital evolution of next-generation autoinjectors – featuring sensors, data logging and wireless connectivity – enables usage tracking, personalised dosing and app integration, aligning with trends in telemedicine and remote monitoring (Figure 4). However, this shift creates technical challenges that require electrically reliable, electromagnetic interference (EMI)-compatible metal components to integrate seamlessly within the electronic architecture.
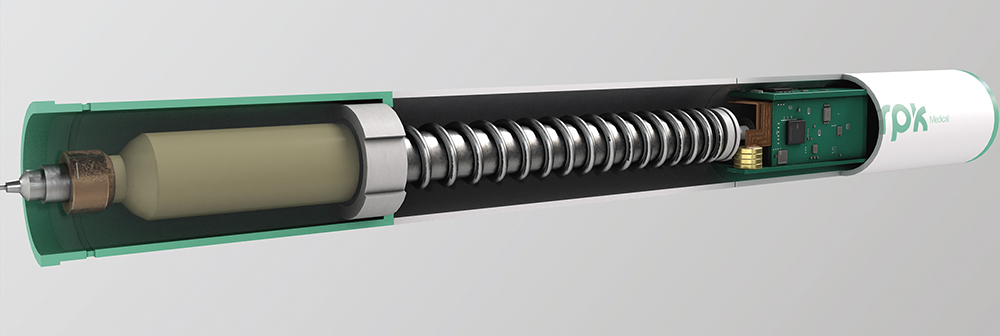
Figure 4: Fully connected autoinjector system.
Traditional elements, such as springs, must now serve additional functions – conducting signals or acting as antennas – while maintaining their mechanical roles. This necessitates careful consideration of conductivity, inductance and shielding. Electrical reliability is critical – issues such as poor contact or corrosion could compromise data transmission, dose logging or even treatment safety. Devices must also resist electrostatic discharge (ESD) from users or the environment, without unintentional activation or damage, placing precision engineering at the core of hybrid, connected systems. Some of the technical challenges include:
- Space constraints amid increased electronic integration
- Demands for durable, stable electrical contacts
- EMI/ESD compatibility requirements.
RPK Medical’s solutions include:
- Low-resistance, high-repeatability contact springs
- Conductive or shielded stamped parts to minimise interference
- Integrated electromechanical subassemblies through automated assembly.
CONCLUSION
These challenges demonstrate that precision mechanics are fundamental to modern autoinjector design. As drug delivery evolves, devices must be as dependable as the therapies they deliver. Collaboration between system designers and component manufacturers enables the development of more efficient, safe and compliant devices. Co-engineering, combined with the use of structural simulation or rapid prototyping, allows early-stage iteration and validation before full-scale production.
The development of next-generation autoinjectors requires innovation not only in electronics and software but also in the mechanical design of their core components. Emerging challenges, including miniaturisation, viscosity, flow control, safety and connectivity, must be addressed holistically, with precision metal engineering playing a central role. The technical solutions outlined in this article – from specialised micro-springs to multifunctional stamped components – demonstrate how tailored mechanics can solve complex problems while enhancing safety and therapeutic performance.
The transition to smaller, connected devices suited for advanced therapies will only succeed if the underlying mechanical challenges are solved. RPK Medical delivers custom metal solutions for each of these challenges, enabling the development of safe and effective devices aligned with the future of personalised medicine. With a global footprint and ISO 13485 certification, RPK Medical is a strategic partner for drug delivery engineering – from design to series production.

