Citation: Pircher D, Zahradník, Ludwig M, “Ensuring Excellence in Manufacturing”. ONdrugDelivery, Issue 178 (Oct 2025), pp 84–90.
David Pircher, Pavel Zahradník and Maxime Ludwig discuss the medical mindset in the context of quality and present examples and tools that are used in practice.

Figure 1: BAUMANN Medical adheres to key principles of zero defects, zero compromises and continuous improvement.
A strong quality culture is the foundation of ensuring consistent product performance and customer satisfaction. Adherence to key principles such as zero defects, zero compromises and continuous improvement is central to maintaining high standards across all operations (Figure 1). When it comes to manufacturing, the focus should be on minimising variations and defects through stringent regulatory compliance, advanced quality control measures and process optimisation. Every employee within the company should be empowered to uphold quality standards, with ongoing training and development supporting this commitment. Risk management should be integrated throughout, using tools such as statistical process control (SPC) and failure mode and effects analysis (FMEA) to proactively identify and mitigate risks.1 This approach fosters a culture of excellence, ensuring that products consistently meet regulatory, safety and customer requirements, while also driving continuous innovation and improvement (Figure 1).
CORE QUALITY CULTURE
BAUMANN Medical’s commitment to delivering high-quality products with minimal batch variation and competitive pricing is rooted in its core values. Consistent quality can reduce the total cost of a product by minimising scrap and rework, directly supporting efficient customer assembly and long-term savings.
This quality mindset is deeply embedded in the company’s culture. Every employee – regardless of role – is empowered to uphold quality standards. In the Medical division, this is reinforced by GMP-compliant environments,2 validated processes and a focus on clean manufacturing. From precision component design to service delivery, quality is integrated into all operations, adhering to ISO 13485 standards.
“BEYOND THE PILLARS OF ZERO DEFECTS, ZERO COMPROMISES AND CONTINUOUS IMPROVEMENT, BAUMANN MEDICAL HAS ESTABLISHED A DEDICATED FRAMEWORK THAT REFLECTS ITS MEDICAL MINDSET AND GUIDES THE PURSUIT OF QUALITY EXCELLENCE.”
Beyond the pillars of zero defects, zero compromises and continuous improvement, BAUMANN Medical has established a dedicated framework that reflects its medical mindset and guides the pursuit of quality excellence.
Employees
Quality emanates from the expertise and dedication of BAUMANN Medical’s team, guided by both corporate and individual values. Committed to medical standards, the company exemplifies a medical mindset to deliver best practices and exceptional service. By prioritising onboarding, continuous training and talent development, BAUMANN Medical fosters a culture of excellence that maintains high standards, ensures seamless services and guarantees top-quality products. This enables the team to deliver exceptional results with responsibility, professionalism and care (Figure 2).

Figure 2: BAUMANN Medical’s employees demonstrate professionalism and care.
Regulatory and Standards
BAUMANN Medical adheres to strict regulatory standards, emphasising product safety through validation processes, digitalised in-process controls (IPC/SPC) and meticulous documentation. The company’s commitment is solidified by compliance with ISO 13485 and 14001 guidelines and regular customer and internal audits. And, of course, it strictly adheres to the appropriate GMP/GDP regulatory standards.
Stability
BAUMANN Medical prioritises precision and stability, maintaining assembly and production stability. The company follows the “Four Eyes” principle for data transfer, batch release and thorough line clearance, as well as adhering to logbook documentation standards. BAUMANN Medical considers the production and specification stability of products to be essential, especially when it comes to supporting the assembly line with high overall equipment effectiveness through its high-quality products.
Risk Management
The company employs a risk-based approach for key elements such as control charts, FMEA and design of experiments. Looking at the bigger picture, BAUMANN Medical is driven by its risk process at all business levels, ensuring that the company is optimally positioned and able to mitigate risks in a situational manner.
ZERO DEFECTS – A COMMITMENT TO EXCELLENCE
First, at BAUMANN Medical, “zero defects” is not just a target – it is a core policy that guides every process. Quality is defined as consistently meeting customer expectations, driven by a relentless focus on excellence in design, manufacturing and service. Through stringent quality controls and advanced technologies, BAUMANN Medical ensures defect-free operations to the highest possible level.
In medical applications, zero defects becomes critical. A minor deviation – such as in a spring for an inhaler or stamping in a continuous glucose monitor – can affect patient safety. BAUMANN Medical uses SPC, real-time monitoring and root cause analysis to detect and resolve deviations before defects occur.
BAUMANN Medical monitors processes in its production SPC through control charts.3 This enables the company to address any issues before the process drifts out of specification. Samples are measured all along the production of an order at regular intervals. This monitors the production output and reacts by correcting the influence parameters on the machine to bring the outputs back towards their target values (Figure 3).
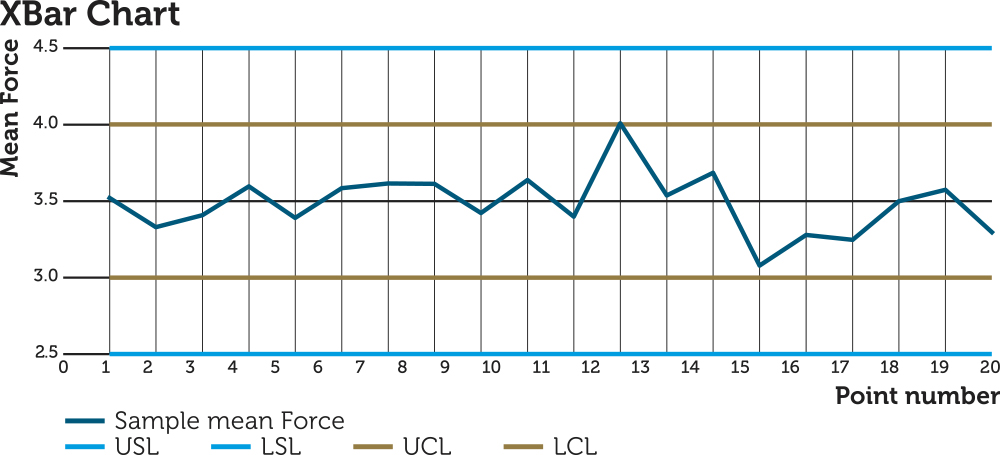
Figure 3: Control chart of force of 100 SPC measurements with sample size five. Point 12 has an average value higher than upper control limit, which triggers correction of the machine settings, and the average value of the next point reverts back to the target average.
“TARGETING A ZERO-DEFECT PRODUCTION, BAUMANN MEDICAL PLACES HIGH SIGNIFICANCE ON ITS SAMPLING STRATEGY FOR PRODUCTION INSPECTIONS.”
Targeting a zero-defect production, BAUMANN Medical places high significance on its sampling strategy for production inspections. The sampling sizes and frequencies are determined by an internal guideline that adheres to VDA 6.3 and ISO 2859 standards. The sampling depends on the project phase, the type of inspection and the criticality of the characteristics. The zero acceptance method is used, which allows optimal sampling sizes – smaller sizes, selected at timely intervals.
As an example, let’s consider a customer who requires an accepted quality level (AQL) of 0.04% on inspection level II for the inner diameter of a spring. The lot size for this product is 20,000 parts. ISO 2859 provides a sampling size of 315 parts to be checked for the whole lot to ensure the target AQL level. A production lot of 20,000 parts of this article requires 20 hours for production. In 20 hours, 10 SPC points are recorded. The inspection plan contains a minimum of 10 x 32 (315/10 = 31.5 (~32)) parts measured to achieve the AQL level. Once a single component fails to meet the specification, the lot is rejected.
ZERO COMPROMISES – REGULATORY AND CUSTOMER REQUIREMENTS, ADHERENCE AND RESPONSIBILITY
The second pillar of BAUMANN Medical’s quality policy is “zero compromises”. The company’s policy is that no compromise is made on key principles at any stage of the product creation process – especially in the areas of quality, safety, compliance, environment and ethics. This means doing things right the first time, even if it is more difficult, time-consuming or expensive. This is how BAUMANN Medical ensures that it adheres to GMP standards within the medical environment. The company starts with unconditional adherence to regulatory requirements, goes above and beyond the 100% compliance standard for customer requirements and, should any cause for deviation arise, follows state-of-the-art customer complaint and notification handling procedures.
BAUMANN Medical makes zero compromises for medical production:
- Adherence to Regulatory: In the medical industry, “zero compromises” also means unwavering adherence to regulatory standards. Whatever is needed to support ISO 13485, US FDA or the EU’s Medical Device Regulation (MDR) approvals for medical devices, BAUMANN Medical can assist with clean and professional documentation that is required from medical device manufacturers. The company’s documentation corresponds to reality – no retroactive filling in and no modification of records.
- Adherence to Customer Requirements: Every customer request and all feedback is evaluated by BAUMANN Medical’s quality teams for feasibility, regulatory compliance and technical impact. Requirements, such as 100% inspection, are integrated into the system and followed without exception, ensuring strict compliance.
- Complaint Handling: For claims and notifications, BAUMANN Medical systematically uses the “8D” problem-solving method, including correction and prevention of reccurrence, lessons learned and best practices shared with other plants. For customer claims or notifications, the BAUMANN Medical mindset to solve the problem sustainably is independent of the type of claim (official or notification).
As an example, let’s imagine that some deformed springs were found in a bag unit. The number of springs found deformed in the bag did not violate the specification. However, BAUMANN Medical built an 8D team (D1) and defined and analysed the problem (D2) following 8D methodology (Figure 4):
- D1 – Form a multidisciplinary team, including different functional experts
- D2 – Define the problem (deformed springs in bag)
- D3 – Containment action to ensure that no parts are affected in production, in storage or at the customer site
- D4 – Analysis of the root causes for occurrence and not detection (in this case, a design optimisation was found in the transport system)
- D5 – Choose and verify permanent corrective actions (design modification of the transport system)
- D6 – Implement permanent corrective actions (implement D5)
- D7 – Prevent recurrence (the design modification was shared with all BAUMANN Medical locations that have similar transport systems)
- D8 – Recognise and celebrate (close the case in form of an 8D report).

Figure 4: BAUMANN Medical follows an 8D methodology to analyse and resolve any manufacturing issues.
CONTINUOUS IMPROVEMENT – DEDICATED CUSTOMER SUPPORT AND ADVANCING THROUGH INNOVATION
The last of the three pillars, continuous improvement, is a key driver at BAUMANN Medical. The company systematically optimises processes, enhances systems and fosters innovation. This is embedded in the company’s culture – every employee is encouraged to contribute, challenge norms and refine even well-functioning processes. With an agile approach to risk and opportunity management, BAUMANN Medical remains flexible and responsive to changing market needs. The company is committed to minimising risk and delivering benchmark-quality products. Robust management systems are developed in line with international standards, legal requirements and customer expectations, and are regularly reviewed to ensure improvement.
Products and Processes
BAUMANN Medical improves processes using data from machines, maintenance and production. Discrepancies and deviations are analysed to find root causes, not just symptoms. Tools such as SPC help to prevent issues early. Procedures are reviewed regularly – what was “good” yesterday may not meet today’s standards. Instructions, layouts and processes are regularly refined. Successful improvements are standardised and shared across sites. Ideas from all levels – operators, technicians and inspectors – provide valuable insights. Continuous improvement, without compromising quality, is part of the company’s DNA – driven by optimisation.
To ensure compliance and continuous improvement, BAUMANN Medical analyses process capability from prototype to series.4 Beyond compliance, process capabilities provide valuable insights for improvement, such as comparisons between machines, raw materials and product ranges.
Figure 5 provides an example of process capability for comparing raw material suppliers of the same article, both being compliant to specification. It shows that Raw Material A has a very centred torque output with a tight distribution. Whereas Raw Material B has a less centred and less tight torque distribution. There is potential for improvement with the supplier of raw material B by implementing actions to tighten and shift the distribution towards the middle of the specification limits (USL, LSL). For such an improvement project, BAUMANN Medical often uses the data-driven “Six Sigma” approach.5
“BAUMANN MEDICAL PROMOTES RISK AWARENESS ACROSS ALL LEVELS, ENSURING TIMELY IDENTIFICATION AND MITIGATION.”
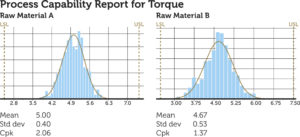
Figure 5: Comparison of raw material suppliers within product specifications.
Risk-Management Processes
Risk management aligns strategy, ensures compliance and enables informed decisions with stakeholders. BAUMANN Medical promotes risk awareness across all levels, ensuring timely identification and mitigation. Integrated with strategy, internal controls and documentation, the company supports audits and regulatory alignment. Standardised processes improve clarity. BAUMANN Medical ensures ongoing risk assessment, giving leadership a global view of critical risks. Participation is mandatory at all sites, with tailored activities and documentation.
One example of how BAUMANN Medical follows a risk-based approach, as required by ISO 13485, is the use of process failure mode and effect analysis (PFMEA) for risk analysis over the whole process. This method allows the company to identify internal risks by understanding the customer’s point of view. It also assists with planning validations for new products by providing a risks assessment on all processes of the article’s manufacture (from the arrival of raw material to shipment of finished goods) and a prioritised list of actions required to mitigate risks in production, both related and unrelated to production processes. PFMEAs are living documents, updated based on learnings in production, engineering and customer feedback.
As an example of an action taken following PFMEA analysis, BAUMANN Medical noticed that a cutting process was lacking stability and detection after receiving customer feedback about a prototype order. The original PFMEA foresaw the risk but did not identify it as high priority. Following customer feedback, the occurrence and detection of the failure assessment was re-rated and action was taken to create a safe launch concept for the product during the validation process with a high focus on the cutting step of the manufacturing process.
CONCLUSION
BAUMANN Medical’s commitment to quality is reflected in its three foundational pillars: zero defects, zero compromises and continuous improvement. By embedding these principles into its culture and operations, the company ensures that it consistently meets the highest standards and remains a trusted partner across industries. With a focus on sustainability, safety and innovation, BAUMANN Medical is well-equipped to thrive in an ever-changing market while continuing to deliver exceptional value to its customers.
Nowhere is this commitment more critical than in the medical industry, where the integrity of each component can directly influence therapeutic outcomes. By applying rigorous standards, design expertise and technical innovation, BAUMANN Medical delivers safe, reliable and high-performance solutions that help shape the future of drug delivery and patient care.
REFERENCES
- “FMEA”. ASQ, 2020.
- “5 main components of GMP: A comprehensive guide”. SciLife, Feb 2025.
- “What is Statistical Process Control?”. ASQ, 2021.
- “VDA 6.3 Volume 4: Process Audit – Statistics (Control Chart and Process Capability)”. VDA QMC, 2021.
- Harry M, Shroeder R, “Six Sigma: The Breakthrough Management Strategy Revolutionizing the World’s Top Corporations”. Crown Currency, 2026.

