To Issue 179
Citation: Jackson L, Hansen T, “From Paper to Planet: Revolutionising Environmental Responsibility in Pharma”. ONdrugDelivery, Issue 179 (Oct/Nov 2025), pp 38–41.
Lori Jackson and Tim Hansen look at the rise of electronic batch records and the role they play in environmental sustainability in the pharmaceutical landscape.
In the current pharmaceutical landscape, environmental sustainability has moved beyond being a competitive edge to become a fundamental expectation. Amid tightening regulatory frameworks, mounting environmental, social and governance (ESG) pressures, and a rapidly evolving digital ecosystem, the pharmaceutical industry finds itself at a critical juncture. One of the most transformative elements of this shift is the rise of electronic batch records (EBRs). More than a tool for quality assurance or operational efficiency, EBRs are now serving as the digital backbone for sustainability efforts across the sector. However, the true power of EBRs is only beginning to emerge with the integration of artificial intelligence (AI), which is redefining what it means to manufacture intelligently, efficiently and responsibly.
“AS DIGITAL MATURITY GROWS AND AI CAPABILITIES BECOME MORE ACCESSIBLE, COMPANIES ARE BEGINNING TO SEE EBRs NOT MERELY AS DIGITAL REPOSITORIES, BUT AS ENGINES OF SUSTAINABILITY.”
Although the concept of EBRs has existed for decades, their adoption has been slow, particularly among CMOs and CDMOs. Barriers such as high implementation costs, complex enterprise resource planning integration and the perceived workflow disruption have slowed progress on this front. Yet, as digital maturity grows and AI capabilities become more accessible, companies are beginning to see EBRs not merely as digital repositories but as engines of sustainability (Figure 1).

Figure 1: Production runs for large-scale commercial packaging can yield lengthy, complex batch records. Manual paper-based processes consume more resources and are not environmentally sustainable.
THE PAPER TRAIL PROBLEM
Historically, batch recordkeeping has been a paperwork-intensive process, with each production run involving extensive documentation, including manufacturing instructions, quality control checks, packaging records, deviation logs, training certifications and more. For large CDMOs, this can translate into millions of pages of printed records annually.
This is not just a storage or labour problem. According to the Environmental Paper Network, 0.9 tonnes of office paper (about 200,000 sheets) consumes approximately 24 trees, 75,708 L of water and 4,100 kWh of energy to produce. This production emits over 2,581 kg of carbon dioxide equivalent (CO2e) when factoring in lifecycle emissions (as calculated by the Environmental Paper Network’s Paper Calculator).
Applying those figures to a mid-sized pharmaceutical manufacturer using 10 million pages annually, the environmental cost includes the destruction of over 1,200 trees, consumption of 3,785,412 L of water and the release of over 225 tonnes of carbon dioxide into the atmosphere.
For a global enterprise operating across multiple facilities, the impact escalates dramatically. Add to that the emissions associated with transporting physical records, the energy used to store and archive them and the inevitable waste from misprints and documentation errors, and it becomes clear – traditional batch documentation is environmentally unsustainable.
ENTER EBRS: SUSTAINABILITY BY DESIGN
EBRs digitise every element of the batch manufacturing process. By eliminating physical paperwork, companies can not only reduce their carbon footprint but also enable broader process improvements.
In 2020, PCI implemented MasterControl’s Qx suite, which was a significant step forward in the company’s digital transformation. Since then, PCI has deployed six modules across 14 of its 17 global sites, as well as successfully training over 7,500 users worldwide. The Qx suite was the foundation for PCI to move forward with EBR. Key environmental benefits from this transition include:
- Elimination of Physical Documents: Each batch record, often hundreds of pages long, is now fully digital. For one product run, PCI consolidated 13 paper batch records supporting 120 stock-keeping units into just two digital production records.
- Reduced Shipping and Storage Emissions: By eliminating the need to transport, archive and retrieve physical documents, PCI minimises emissions associated with document handling logistics.
- Fewer Reprints and Errors: Digital workflows mean fewer batch failures due to paperwork errors, reducing rework and material waste, which is a notable environmental gain in high-volume manufacturing.
- Less Hardware Waste: As legacy systems are consolidated, redundant servers, scanners and printers are phased out, reducing e-waste and power consumption.
“PCI’S COMMITMENT TO ENVIRONMENTAL STEWARDSHIP GOES BEYOND COMPLIANCE – IT IS ABOUT BUILDING SMARTER, LEANER AND MORE SUSTAINABLE SYSTEMS.”
PCI’s commitment to environmental stewardship goes beyond compliance – it is about building smarter, leaner and more sustainable systems. EBRs eliminate waste at the source, laying the foundations for advanced tools like AI and predictive analytics that will redefine sustainable manufacturing.
EBRS AS A CATALYST FOR SYSTEMATIC DIGITAL ACTION
As the pharmaceutical industry continues its shift towards sustainability and operational excellence, the advancement of Pharma 4.0™ offers a powerful lens through which to view this transformation.1 According to the ISPE 7th Pharma 4.0 Survey (2023), organisations are rapidly progressing beyond early-stage planning and into actionable, integrated digital practices. The proportion of companies reporting they had “not started” with Pharma 4.0 initiatives dropped from 31.2% in 2021 to 15.1% in 2023. Simultaneously, those engaged in “pilots” or “systematic ongoing actions” rose significantly, now representing 58.1% of respondents.
This upwards maturity trend represents an industry-wide commitment to embedding digital tools into the very core of pharmaceutical manufacturing. The report shared that 50% of manufacturing and engineering respondents identified EBRs as a main focus area and foundational for achieving operational consistency, regulatory compliance and sustainability.
EBRs exemplify the type of digitisation that transitions Pharma 4.0 from aspiration to execution. Digitised systems reduce the dependency on paper, streamline batch record review cycles, improve deviation handling and ensure real-time data traceability, all of which contribute to enhanced process control and resource efficiency. For organisations moving from pilot to systematic implementation, EBRs are often among the first large-scale digital tools to be operationalised, offering an immediate return on investment in terms of quality and operational agility.
By embedding EBRs into their transformation roadmap, pharma companies are not just digitising records, they are laying the groundwork for a more integrated, sustainable and intelligent future of manufacturing (Figure 2).
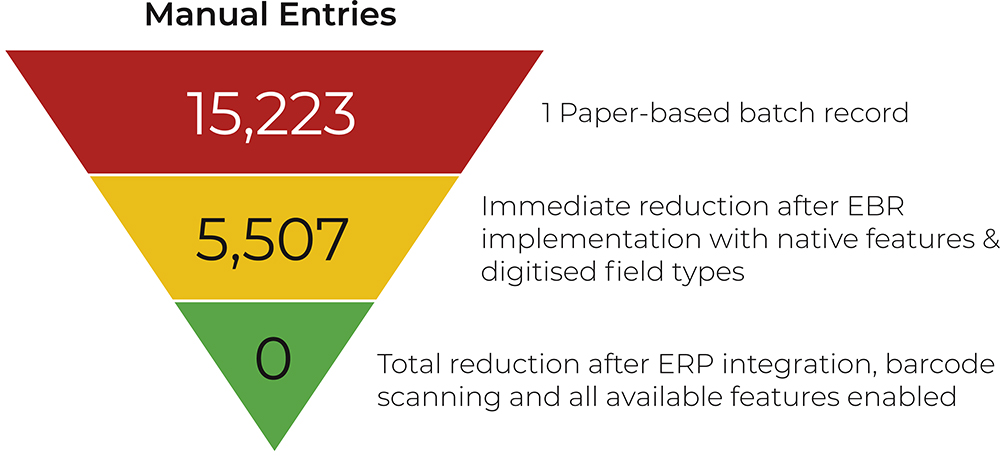
Figure 2: One batch record required over 15,000 manual entries, but that number immediately reduced upon EBR implementation.
DIGITAL FOUNDATIONS ENABLE AI AND OPTIMISATION
While EBRs provide the structure and data integrity needed for compliance and efficiency, AI adds the intelligence layer that unlocks continuous improvement. Through the application of machine learning, predictive analytics and natural language processing, pharmaceutical manufacturers can convert vast repositories of batch data into actionable insights. AI can identify patterns and correlations that would be nearly impossible to detect manually, enabling a shift from reactive to proactive manufacturing.
For example, AI algorithms can analyse digital batch records to detect deviations in real time and even predict potential process failures before they occur. This not only reduces downtime but also minimises the risk of batch failures, which are notoriously resource intensive. Every failed batch means wasted raw materials, lost production time and unnecessary energy consumption – all of which contribute to a larger environmental footprint. By catching these issues early, AI can help ensure that manufacturing runs more smoothly and sustainably.
AI also enables optimised material usage. Historical data can inform smarter formulation decisions, helping manufacturers to reduce overages, balance inputs more precisely, and minimise solvent and water use. These micro-optimisations, applied consistently across thousands of batches, result in macro-level environmental savings. Moreover, the use of AI to fine-tune manufacturing parameters can improve yield and reduce the frequency of out-of-spec batches, further cutting waste.
“PCI IS CURRENTLY TESTING AI-POWERED TOOLS FOR AUTOMATED DOCUMENT TRANSLATION AND EXAM GENERATION, SIGNIFICANTLY REDUCING THE TIME AND COMPLEXITY INVOLVED IN ONBOARDING STAFF ACROSS ITS GLOBAL SITES IN GERMANY AND SPAIN.”
In addition to process optimisation, companies such as PCI are using AI for operational efficiency in training and global compliance. PCI is currently testing AI-powered tools for automated document translation and exam generation, significantly reducing the time and complexity involved in onboarding staff across its global sites in Germany and Spain. These technologies lower the language and training barriers that often slow digital adoption, ensuring more rapid deployment of sustainable practices.
CHALLENGES: TIME, RESOURCES AND CULTURE
Of course, implementing EBR systems and layering AI on top is not without its challenges. Time and resources, rather than resistance to change, are the primary barriers to adoption. For companies navigating frequent mergers and acquisitions, harmonising systems across facilities is particularly complex. PCI has addressed this by avoiding a “big bang” roll-out and instead adopting a modular approach, tailoring onboarding checklists and deployment strategies to the unique needs of each site. This phased methodology has proven effective in reducing disruption while still moving the organisation steadily towards full digital maturity.
REGULATORY AND STRATEGIC ALIGNMENT
From a regulatory standpoint, the shift towards digital documentation is also gaining traction. Guidelines such as the US FDA’s 21 CFR Part 11 and the EMA’s GxP reflection papers strongly advocate for electronic systems that support data integrity, real-time traceability and audit readiness. EBRs meet these criteria by offering tamper-evident digital trails, time-stamped approvals and secure storage, reducing the risk of incomplete or lost records.
What is particularly compelling is how this regulatory alignment dovetails with environmental goals. As pharmaceutical companies face increasing scrutiny from investors, regulators and consumers alike, tools that enhance both compliance and sustainability offer a rare and valuable convergence of interests. The ability to demonstrate traceable, verifiable environmental responsibility is becoming just as important as showing product quality or regulatory adherence.
LOOKING AHEAD: A GREENER PHARMA INDUSTRY
The integration of EBRs with broader enterprise systems promises even greater impact. PCI envisions a future where EBRs connect seamlessly with ERP platforms, laboratory information management systems and environmental monitoring systems. This kind of connectivity will enable real-time carbon tracking, dynamic resource allocation and continuous improvement loops driven by AI. Instead of reacting to inefficiencies after the fact, companies will be able to course-correct in real time, reducing their environmental impact with each batch produced.
EBRs are no longer just digitised file cabinets, but are foundational elements of a smarter, more sustainable pharmaceutical manufacturing model. When augmented with AI, EBRs become powerful tools for process optimisation, environmental stewardship and strategic alignment. As companies like PCI lead the way, it is becoming increasingly clear that the path to a greener industry is not paved with paper. It is driven by data, powered by intelligence and guided by a deeper commitment to both operational excellence and planetary health.
REFERENCE
- Minero T, Kuger L, “The 7th Pharma 4.0™ Survey: Digital Transformation”. ISPE, Sep–Oct 2024.

