To Issue 148
Citation: Bhat J, Dalvi M, “Hard Capsules: a Holistic Approach for Oral Drug Delivery and Formulation.” ONdrugDelivery, Issue 148 (May/Jun 2023), pp 28–31.
Jnanadeva Bhat and Manali Dalvi discuss how advances in capsule technology as an oral drug delivery method have opened up new avenues for the development of more effective and patient-friendly drugs.
“Advancements in oral drug delivery have overcome many of the challenges once associated with it.”
In recent years, the pharmaceutical industry has seen a surge in innovation. The convergence of existing trends involving the need for complex delivery systems, together with significant growth in comprehensive product development, has modified the landscape of drug discovery on a global scale. In parallel, the industry is witnessing numerous emerging technologies such as genomics, nanoscience and biotechnology.
The industry is constantly striving to improve drug delivery systems to enhance the therapeutic efficacy of drugs, reduce side effects and improve patient compliance. Driven by increased investment in R&D, expiring patents in the pipeline and consistent regulatory support, the pharmaceutical industry is undergoing a dramatic shift away from many of its conventional processes.
Researchers are now focusing on continuous and incremental innovation in pharmaceutical formulations to develop new products with minimal side effects, while also providing targeted and specific therapeutic breakthroughs. The industry is exploring various dosage forms and carrier systems to accelerate development and tailor development to evolving trends in delivery methods, such as tablets, capsules, injections, transdermal patches, inhalers and implants. Each of these delivery methods has its own set of challenges and benefits, depending on the drug and target delivery site. Therefore, the delivery system should be selected on a case-by-case basis, taking into account factors such as drug solubility, stability and bioavailability.
An oral drug delivery system (ODDS) is considered the most attractive delivery format due to its convenience, patient preference and cost effectiveness. According to global estimates, approximately 60% of commercially available small-molecule drug products are administered orally.1 The oral route of drug delivery has higher patient compliance than parenteral routes, such as intravenous, subcutaneous and intramuscular injections. It allows for the delivery of a wide range of therapeutic ingredients. However, some of these may cause challenges due to their physicochemical properties, such as poor water solubility and membrane permeability, as well as gastric irritation – making it difficult to develop effective oral dosage forms. However, advancements in oral drug delivery have overcome many of the challenges once associated with it.
“There is a shift away from the traditional powder-filling technique and new possibilities using capsule shells as delivery containers are opening up.”
HARD CAPSULES AS A GO-TO OPTION FOR DRUG DELIVERY
Lately, there have been numerous innovations in oral drug delivery. The sector is expanding to encompass areas including smart drug delivery, 3D printing technology and nanotechnology-based drug delivery – which all align with the trend towards customised healthcare. ODDS options include tablets, capsules, powders, oral films and liquid orals.
Tablets and capsules are two popular dosage forms, with hard capsules catering more to the industry’s needs. Owing to their simplicity, ease of manufacturing and patient acceptance, they are the preferred dosage form over tablets. Unlike tablets, capsules do not require binders or further complex granulation processing, which can increase the cost of production and require stricter quality control. They can be filled with a variety of formulations, including powders, pellets and liquids, making them a versatile dosage form. They also have the advantage of being able to deliver a variety of doses without the need for specialised equipment. This versatility facilitates easier development and customisation of formulations – essential for meeting the diverse needs of patients.
BEYOND HARD CAPSULES
The use of capsules in drug development is emerging as a promising area of exploration for the industry. There is a shift away from the traditional powder-filling technique and new possibilities using capsule shells as delivery containers are opening up. This is becoming an all-encompassing solution for all forms of delivery, such as solids, liquids and semi-solids, and where factors such as flowability, content uniformity, customised drug product development, patient compliance, efficacy and stability enhancement need to be considered.
Capsules have revolutionised drug delivery by allowing multiple APIs to be delivered together without compatibility challenges. This is achieved by encapsulating the various actives in different ways in a single or dual capsule shell. The dynamic nature of hard capsules enables them to server as a carrier for various modified release APIs. Additionally, it allows for the delivery of multi-particulate dosage forms, such as pellets and granules, coated with functional polymers to achieve modified-release properties. It also facilitates the combined encapsulation of mini tablets or multi-unit pellet systems, thereby controlling the release of the API(s).
Furthermore, advances in capsule technology have enabled the encapsulation of liquids, along with a tablet, pellet or capsule, inside. This not only improves the bioavailability of APIs but also enables customised release – either at specific times or targeted to an exact area of the body – to enhance the therapeutic effect of different molecules.
CAPSULES AS A ONE-WAY SOLUTION
The pharmaceutical industry is constantly evolving and exploring new ODDS possibilities. However, there are some limitations in the process. Oral delivery may present challenges relating to bioavailability, stability and efficacy. Advancements in capsule development are now addressing factors such as incompatibility, solubility, efficacy enhancement and content uniformity (Figure 1).
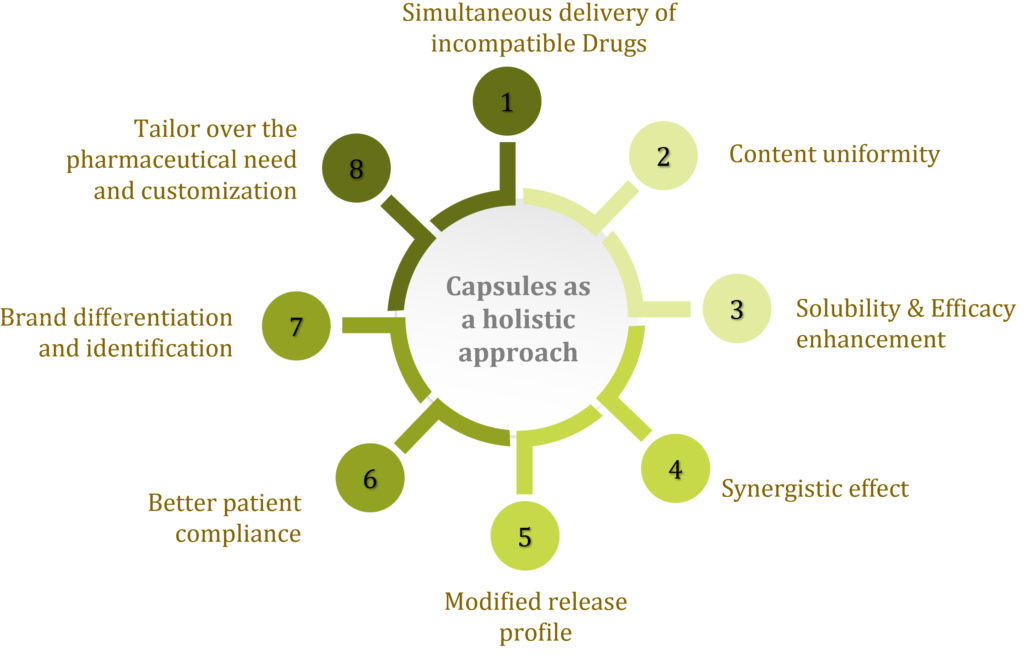
Figure 1: Capsules as a holistic approach for an oral drug delivery.
The novel capsule delivery format enables the distribution of the APIs in two major formats:
- Solid–solid combination
- Solid–liquid combination.
“Combination ODDSs that incorporate different release profiles in a single capsule can offer several advantages in terms of treatment customisation, patient compliance, convenience and therapeutic outcomes.”
SOLID–SOLID COMBINATION
Combination Drug Delivery and Synergism
Combination drug delivery is a growing trend, especially with the expiration of patents and the increase in generic products. It has demonstrated its effectiveness when it comes to treating diseases such as cancer, diabetes and cardiovascular disease. This strategy of combining different drugs with different mechanisms of action can lead to a synergistic effect with better treatment outcomes. It enables the constituent drugs to function together to target multiple pathways that contribute to the disease, offering an overall enhancement to the therapeutic effect.
With the advancement in capsule-filling technology, it is now possible to encapsulate multiple APIs in various forms, including powder, pellets, tablets and mini-tablets. (Table 1). This assists in a quick changeover to alternative combinations. This approach to drug development increases patient adherence, reduces multi-drug resistance with single-capsule administration and minimises the chance of taking the incorrect combination or an improper dose of the API. Moreover, it also improves the content uniformity of the product.
| Solid–Solid Combination | Conceptual Examples |
| Powder in capsule (conventional) | Amoxicillin/Rifampicin/Gabapentin |
| Pellets in capsule | Omeprazole DR/Lansoprazole DR |
| Pellets of multiple APIs in capsule | Carvedilol phosphate IR + Carvedilol phosphate DR |
| Pellets and tablet in capsule | Domperidone tablet + Omeprazole pellets/ Levosulpride SR tablets + Rabeprazole ER pellets |
| Powder and tablets in capsule | Trospium IR as powder + Trospium SR as tablet |
| Powder and pellets in capsule | Carbidopa IR as powder + carbidopa DR as pellets |
| Micro-tablets in capsule | Venlafaxine tablet |
| Capsule in capsule | Pre and probiotic + alpha lipoic acid & vitamins |
| Solid–Liquid Combination | Conceptual Examples |
| Liquid fill capsule | Ibuprofen/ peppermint + spearmint oil |
| Tablet in liquid fill capsule | Atorvastatin + Aspirin enteric-coated tablet |
| Capsule in liquid fill capsule | Domperidone Maleate + Rabeprazole DR pellets in tiny capsule |
| Pellets in capsule in liquid fill capsule | Domperidone Maleate + Omeprazole DR pellets |
| Single API pellets in liquid fill capsule | Artemether + Lumefantrine pellets |
Table 1: Combinations for hard-capsule filling.
Overruling the Incompatibility Challenge
Incompatibility between pharmaceutical ingredients is a significant challenge encountered during the development of drug products. Reactive impurities produced by drug–drug interactions may cause product instability due to the production of toxic degradants, which can limit the scope of product development. Capsules provide a solution to this problem by allowing the delivery of multiple APIs without causing compatibility issues. By using a protective coating, the formulator can encapsulate multiple compatible or incompatible APIs in a single capsule.
Aside from that, a capsule-in-capsule approach is the best solution for resolving compatibility issues. This technique allows the encapsulation of APIs in two separate capsules. Physical separation of the two APIs by the inner capsule decreases the risk of interaction, providing a protective barrier (Figure 2). This separation helps maintain the stability and integrity of the individual APIs until they are released and reach their target site.
Figure 2: Solid–solid capsule combinations.
Modified-Release Patterns
Combination ODDSs that incorporate different release profiles in a single capsule can offer several advantages in terms of treatment customisation, patient compliance, convenience and therapeutic outcomes. Various release profiles, such as immediate release (IR), delayed release (DR) and sustained release (SR), can all be achieved in a single capsule by using pellets with modified-release properties within the coating processes.2
Moreover, coating pellets with different polymers is a crucial step in this type of ODDS. This involves applying a polymer layer onto the surface of the drug pellets, which provides a barrier that controls the release of the drug. Coating can be achieved via several methods, such as fluidised bed coating, pan coating or spray coating. These techniques ensure precise control over the thickness and composition of the coating layer, which ultimately determines the drug-release characteristics. However, selecting the correct polymer and coating technique is essential for controlling release kinetics in the pellets.
The combination ODDS can provide multiple release profiles in a single dosage form, by encapsulating the coated pellets within a capsule shell. These profiles include immediate release with rapid onset, delayed release allowing the release of the drug after a predetermined lag time, and sustained release providing a prolonged release over an extended period. Mini- or micro-tablets in capsules provide another approach for release profile modification, allowing for a higher variation in the release profile compared with the pellets due to the surface area of the inner dosage form. Although not a conventional drug delivery method, this strategy opens up customised dosing regimens, with increased therapeutic efficacy and decreased dosing frequency to improve patient convenience.
SOLID–LIQUID COMBINATION
Encapsulation technology is no longer restricted to solid formats but now also can include liquid formulations.3 Capsules are now widely used for the delivery of various non-solid formulations, such as non-aqueous liquids, suspensions, self-emulsifying drug delivery systems, thixotropic gels, semisolids and hot melts. After filling, the capsules are sealed with an appropriate sealing technique to prevent leaking.
There are four major combinations possible with this technique – tablet in liquid, capsule in liquid, pellets in capsule in liquid and pellets in liquid (Figure 3). These combinations allow the formulator to develop their product with a multiple release profile without changing the original form of the API (solid or liquid) (Table 1). For example, a single API in a liquid form (IR) and another API in a tablet or capsule form inside the outer capsule (DR/SR). This delivery format enables the delivery of hygroscopic APIs.
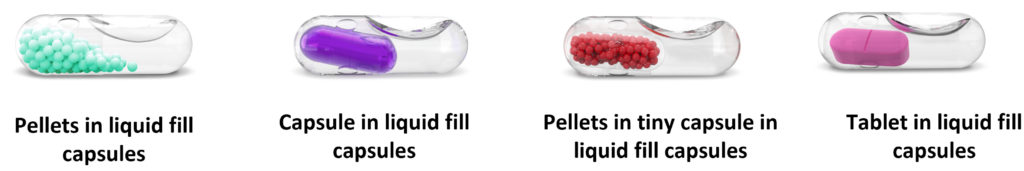
Figure 3: Solid–liquid capsule combinations.
As these materials are very sensitive to moisture, their delivery can be facilitated by encapsulating the material with molten excipients, such as polyethylene glycol or wax up to a temperature of 70°C. These formats are emerging as an effective way to deliver highly potent drug formulations, such as anti-cancer treatments, steroids, hormonal therapies and immunosuppressants. This technology has also made it easier to administer an exact amount of potent drugs by suspending or solubilising it in the carrier liquid, thereby avoiding any loss of the potent formulation. Moreover, given the flexibility in dose adjustment between the inner solid component and the outer liquid, this technique facilitates customised treatment and dose titration based on patient needs.
CONCLUSION
Overall, advances in capsule technology as an oral drug delivery method have opened up new avenues for the development of more effective and patient-friendly therapeutics. Techniques such as capsulein- capsule, combination filling, modified release, functional coating and 3D printing of capsules are gaining momentum in the market. These advancements have provided numerous benefits in terms of combating stability and compatibility issues, as well as helping to manage delivery challenges. They have also enabled brand differentiation, helping to support increased patient compliance. Continued research will likely progress towards myriad future advancements in oral drug delivery.
REFERENCES
- Alqahtani M et al, “Advances in oral drug delivery”. Front Pharmacol, 2021, Vol 12, Article 618411.
- Hiew T et al, “Understanding the release performance of pellets with hydrophobic inclusions in sustained-release coating”. Int J Pharm, 2019, Vol 557, pp 229–237.
- Biyani M, “Selecting excipients for liquid-filled Hard capsules”. Pharm Technol, 2018, Vol 30(8), pp 16–19.

