Citation: “Interview: Steven Kaufman. Delivering the Future – Inside the World of Autoinjector Innovation”. ONdrugDelivery, Issue 178 (Oct 2025), pp 130–133.
Q How have you seen drug delivery devices evolve over the course of your career and where do you believe things are heading in the next decade?
A Across my career, I’ve worked with several great companies and witnessed the growth of the industry firsthand. From my perspective, two of the most significant changes are, first, people are more knowledgeable across the industry when pairing their primary container with the right device and final assembly solution and, second, pharma evaluates device technologies much earlier today than they did a decade ago and are willing to invest when it makes sense to do so.
The latter is a profound shift. Historically, pharma companies would wait until the end of Phase II or early Phase III trials, or even until after launching in a prefilled syringe (PFS) before thinking about a device solution. Today, both pharma and biotech, large and small, select device platforms much earlier in their development cycle and hire staff or use consultants that are from the device space and integrate them into their teams – which I think is a positive move.
Another shift I’m seeing is that people are starting to focus more on device differentiation. For example, as interim CCO at Portal, I introduce the PRIME Nexus™ smart reusable autoinjector regularly to potential customers and get to hear feedback firsthand (Figure 1). There is a strong attraction to sustainability and flexibility in terms of delivery envelope, as well as having device solutions that are economical. These are all common themes in many of my discussions.

Figure 1: Portal’s PRIME Nexus™ reusable autoinjector.
“I BELIEVE THAT WE ARE AT A TIPPING POINT WHERE THE INDUSTRY IS STARTING TO LOOK AT NEW DEVICES DIFFERENTLY AND TO BE OPEN TO CHANGES.”
I believe that we are at a tipping point where the industry is starting to look at new devices differently and to be open to changes. So far, we’ve seen incredible success from the major platform autoinjector devices like Molly (SHL Medical) and YpsoMate (Ypsomed), which have replaced a lot of the time-consuming bespoke customised device solutions. They are great devices, and I have a lot of respect for both companies. And now we’re starting to see some of the other disposable autoinjector platforms – and reusable autoinjectors – garnering attention. I think that’s pointing to where the industry could be heading in the future.
In the very near term, we’re probably going to be staying with a lot of the same types of traditional devices – more platforms, more single-use disposable – but with the issues of higher viscosity and different fill volumes, and different primary containers, it is very difficult for one device to suit all those needs. At the same time, sustainability continues to be a very significant topic, particularly in Europe and in the context of glucagon-like peptide-1 agonists (GLP-1s), for example. Now, globally, so many devices are used daily for GLP-1 treatment and that number continues to grow.
Q You touched upon sustainability. Considering current industry trends, how can drug delivery device companies help minimise waste and reduce overall treatment costs as well?
A One of the big things is that, as an industry, we’ve gone from making millions and tens of millions of autoinjectors a year, to over a billion a year. When this many autoinjectors, and drug delivery devices in general, are being disposed of, a lot of people start to wonder “Is this the right approach?” – both from a sustainability and a cost perspective.
“PRIME NEXUS™ AND OTHER REUSABLE AUTOINJECTORS COULD HAVE A UNIQUE OPPORTUNITY TO HELP PHARMA COMPANIES TO FIND A COST MODEL THAT WORKS MUCH BETTER THAN THE CURRENT DISPOSABLE PARADIGM.”
PRIME Nexus™ and other reusable autoinjectors could have a unique opportunity to help pharma companies to find a cost model that works much better than the current disposable paradigm. For example, if you’re on a GLP-1, you’re going to be using roughly 156 autoinjectors over three years, all of which are disposed of in a sharps container. Think about all the waste and cost that generates.
Compare that with a reusable device where, at least in PRIME Nexus™’ case, we have a single device that is good for around three years of use. It sits in your home, very much like an electric toothbrush on a docking station, charged and ready to go. The disposable part is a relatively small cassette that inserts in the reusable device. These cassettes are designed for 1 and 2.25 mL PFSs (with 5.5 and 10 mL PFSs under development), which brings huge advantages from a flexibility perspective – but also radically reduces the environmental impact.
The key, though, is that the price point for the reusable has to be at an affordable level. At Portal, we believe that with the current device and the cost structure, you’re paying back the cost of the device within six months from the per-dose savings. Then you still have it for another two and a half years.
I’m not saying that price is the only consideration – sustainability is important, flexibility is important – but achieving that balanced approach to the cost makes it easier for companies to convert from the standard disposable platform autoinjector model.
Q Looking to the future, what key features should every autoinjector have – both in terms of entry level requirements and, further than that, what separates the very best from the rest?
A To answer the second part first, I think what differentiates the best from the rest is the ability to think through the entire value chain – to understand the fill-finish side, the primary container side, the device side, the manufacturing side and the final assembly, labelling and release. The most successful companies are ones that understand the full value chain and can support decisions throughout the entire journey. There are plenty of companies that can assist with connectivity, human factors, small- to large-scale final assembly, primary container verification or feasibility studies – but ultimately you need the knowledge and experience to bring that all together. At Portal, we pride ourselves with having a strong team with a supportive partner structure. We are proud of our relationship with Gerresheimer (Düsseldorf, Germany) as our strategic partner for the commercialisation of the PRIME Nexus™ device, which was recently made public (Figure 2).
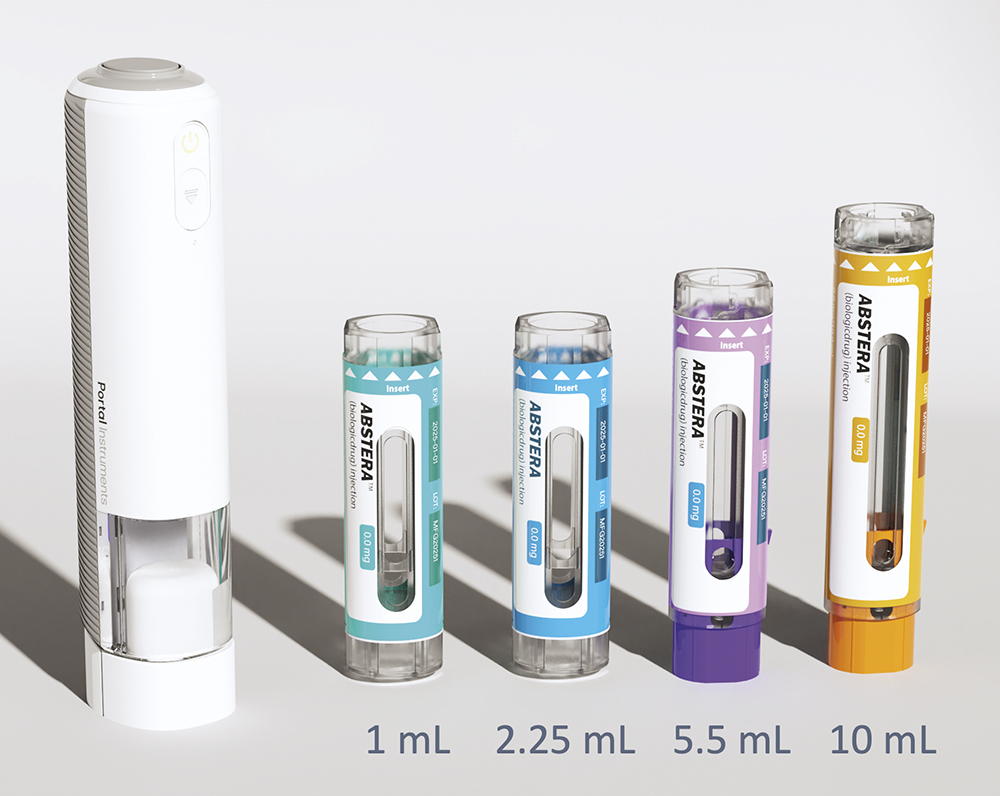
Figure 2: The PRIME Nexus™ reusable smart autoinjector is suitable for 1 mL and 2.25 mL PFSs (using the same cassette) and the 5 mL and 10 mL PFS versions are under development.
Going back to the first part of the question, I think – at a bare minimum – devices need to be what I often call a “hybrid” design. I may be biased, but I like the Aidaptus device (Owen Mumford in partnership with Stevanato Group), which accommodates both 1 and 2.25 mL PFSs in one form factor, another example is Eco-inject a company that has also has a hybrid autoinjector using sustainable materials for both 1 and 2.25 mL PFSs. PRIME Nexus™ is the same in principle, using hybrid cassette designs with no metal parts and a low component count that can hold 1, 2.25, 5.5 and 10 mL PFSs, but the main device itself is reusable, and I think that such devices have other distinct advantages.
Beyond having the flexibility to facilitate different primary containers, I would say that autoinjectors need to be able to deal with high variance in viscosity and changes in fill volume. For PRIME Nexus™, we were originally scoped out handling up to 50 cP, but we’ve performed studies where we effectively delivered in the hundreds of centipoise. I think it’s important to expand the performance envelope of new devices beyond what we have currently in the market, and I can see that biotech and pharma companies are starting to expect that device companies can do this.
Q How important are connectivity and digital health for developing combination products for the delivery of novel biologics?
A The core appeal of digital health is its potential to help address human factors and patient adherence concerns. There’s a significant opportunity to improve onboarding and the general patient experience here, which may be critical to tackling the industry’s long-standing challenges with adherence. Another factor that comes to mind is that digital health can also be a source of data for patients and biopharmaceutical companies, which can give them a better chance of getting their products to market, so there’s a lot of value there as well. At Portal, our current focus is to just keep carrying out feasibility studies to keep demonstrating the connectivity technology to potential partners. And if a customer would like to implement the technology, we can easily add that to the device with the support of our partners like Gerresheimer.
Q How does Portal employ its extensive experience in combination product development across regulatory, industrialisation and commercialisation to help pharma and biopharma companies de-risk and accelerate their drug device combination programme?
A It’s true that across our team at Portal – the executives, the board and all the people that are working to make the device and performing the studies – we have the required device development knowledge from working with electromechanical solutions for more than a decade and there’s a lot of expertise within our teams and from our partners. We pride ourselves on being extremely flexible and nimble, which is possible because we’re a relatively small company. That flexibility helps us really use our collective experience to try and ensure that patients are properly considered when our partner pharma companies are looking at different options. We have that institutional knowledge across quality, regulatory, commercial and R&D.
“WHAT IS PARTICULARLY EXCITING IS WHEN WE CAN SHOW THAT THINGS THAT HAVE BEEN CONSIDERED CHALLENGING ARE NOW POSSIBLE WITH NEXT-GENERATION DEVICES, SUCH AS DELIVERING FORMULATIONS OVER 100 CP.”
What is particularly exciting is when we can show that things that have been considered challenging are now possible with next-generation devices, such as delivering formulations over 100 cP, as I mentioned earlier. Working with pharma companies, we can use our experience to guide them and recommend certain primary containers, or we can give them feedback from what we’ve seen from our human factors studies. On that front, we’ve started to work with Interface Analysis Associates (Saratoga, CA, US), Kymanox and others, and we have been running several usability studies to do more in that area ourselves in-house.
Our experience has also allowed us to apply consistent underlying principles to the design of PRIME Nexus™. We’ve made sure that it’s flexible but cost effective at the same time. To reiterate a couple of the key points, it’s about the ability to deal with multiple fill volumes and different viscosities within the same platform and being able to maintain almost the same injection time regardless. Injection time has come up a lot in our conversations recently. We can fine tune the delivery parameters based on the needs of the formulation. And then, we can lean on our experience again to help with clinical studies or during drug development where it’s still unknown what the final fill volume will be.
With automatic needle insertion and retraction and the versatility of the platform, PRIME Nexus™ has unprecedented flexibility. You don’t have to make any changes to the hardware because the software can easily be updated and changed, which is possible due to PRIME Nexus™ being an electromechanical device. And, if I may say so, the device is designed to look elegant as well, which I’m a big advocate of.
I’ve been asthmatic since I was 10, and when I was a kid I used to feel the need to hide my inhaler because I didn’t want the other kids knowing I had an issue. So, over the course of my career, I’ve tried to work with companies that want to make devices that are not only effective and great for patients but that are also designed to not look so distinctively medical, and that by doing so we can help to remove the stigma associated with taking medication.
I think one mistake that people make with electromechanical devices, be they on-body injector or autoinjector, is to try to make them too complex and include too many bells and whistles. We have to provide options, but at the same time, we need to keep them simple.
If we can achieve that simplicity, cover the whole value chain with our partners, help the environment and find a cost-effective balance, I think that devices like PRIME Nexus™ have a real opportunity to influence the market and provide significant benefit to pharma companies and patients alike.

