Citation: Vasiev A, Miller E, “Autoinjectors Versus On-Body Systems: The Future of Large-Volume Delivery”. ONdrugDelivery, Issue 178 (Oct 2025), pp 124–129.
Dr Alex Vasiev and Dr Ethan Miller weigh up the benefits and costs of using on-body injectors versus autoinjectors, discussing delivery, design and patient population variability alongside recent advances in on-body injector development.
With the continuing shift of healthcare from clinics to the home, more injectable therapies are moving from intravenous (IV) to subcutaneous (SC) administration, particularly in oncology and immunology. SC delivery of high-dose biologics offers clear advantages, including greater patient convenience and potential cost savings, but large-volume or high-viscosity formulations bring distinct challenges in formulation stability, device design and the route to commercialisation.
“AS DELIVERY TECHNOLOGIES ADVANCE, THE QUESTION IS NO LONGER WHETHER LARGE-VOLUME PARENTERAL THERAPIES CAN BE ADMINISTERED OUTSIDE THE CLINIC, BUT HOW TO DO SO MOST EFFECTIVELY. AUTOINJECTORS HAVE HISTORICALLY EMPOWERED PATIENTS TO SELF-ADMINISTER AT HOME, BUT THEIR PRACTICALITY DIMINISHES AS VOLUMES RISE BEYOND 2 ML.”
As delivery technologies advance, the question is no longer whether large-volume parenteral therapies can be administered outside the clinic, but how to do so most effectively. Autoinjectors have historically empowered patients to self-administer at home, but their practicality diminishes as volumes rise beyond 2 mL.
Debate continues across the industry on key considerations such as patient experience, cost and reimbursement, device complexity, regulatory uncertainty and the level of investment required for new infrastructure. A growing consensus suggests that large-volume delivery strategies may increasingly consolidate around wearable on-body injectors (OBIs) rather than oversized autoinjectors. As the adage goes: just because it can be done does not mean that it should.
THE HANDS-FREE ADVANTAGE
OBIs are inherently more complex and costly than autoinjectors. They often incorporate adhesive pads, multiple components and sometimes reusable elements, electronics and software. These features increase development costs, extend timelines and place higher demands on both supply chains and users. Autoinjectors, in contrast, are simpler and more cost-efficient. However, when used for volumes exceeding 2 mL, they require greater power and enhanced user interfaces to accommodate longer injection times, as well as larger, heavier form-factors that erode their perceived advantages.

Figure 1: Large-volume autoinjectors require increased power, posing a technical challenge; however, the primary barriers remain usability issues and the pain associated with rapid delivery of large volumes.
Both device types also raise reliability considerations. OBIs must address risks such as occlusion, leakage and incomplete dosing during extended wear, whereas large-volume autoinjectors must ensure the long-term stability of their high-power mechanisms and maintain the integrity of novel container closures.
The primary limitations of large-volume injections stem from human physiology. Autoinjectors are generally well tolerated for small volumes (<2 mL),1,2 but rapid administration of larger volumes can strain SC tissue, elevate interstitial pressure and increase discomfort. Attempts to reduce injection volumes by concentrating formulations can introduce additional challenges – higher viscosity solutions require more power to deliver effectively (Figure 1).
OBIs overcome many of these limitations by enabling hands-free, extended delivery over minutes to hours, which significantly reduces power requirements (Figure 2). Slower, controlled administration decreases tissue stress and interstitial pressure, improving overall tolerability. Studies, including that by Doughty et al (2016),3 demonstrate that extended delivery can substantially reduce perceived pain. This extended delivery time therefore not only enhances patient comfort but also provides greater dosing flexibility and a broader operational envelope, which is a benefit in early clinical evaluation.
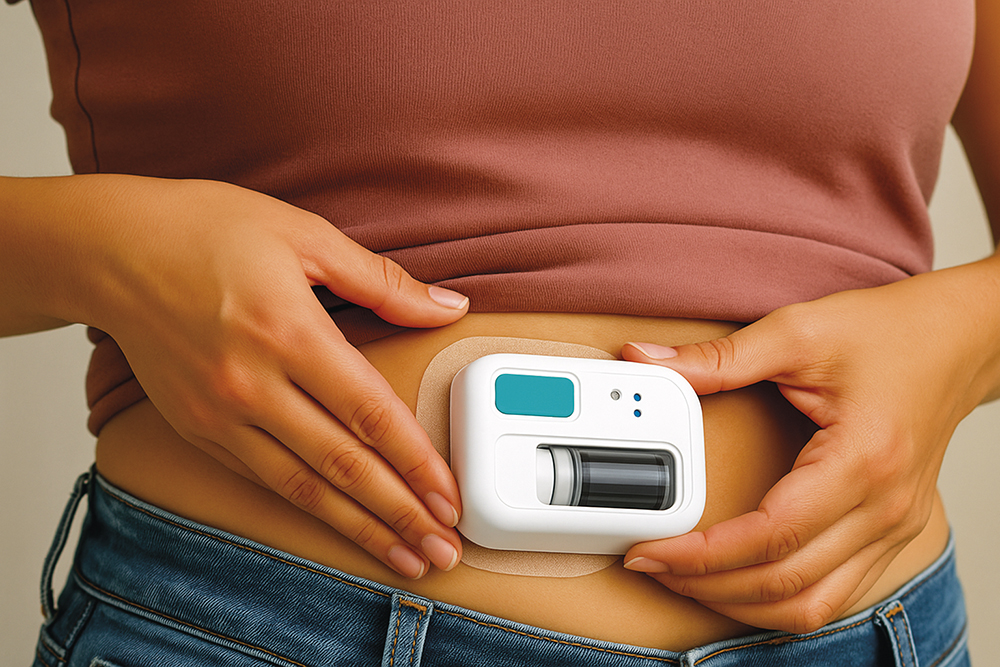
Figure 2: Wearable technologies are becoming the established solution for the delivery of high-dose (large-volume or high-viscosity) formulations.
DEVICE AND CLINICAL STRATEGY
SC absorption is influenced by injection volume, viscosity and administration site, making device selection critical to clinical success. Early-phase studies (Phase I/Ib) evaluate a range of injection volumes, formulation concentrations and delivery rates to characterise pharmacokinetics, systemic exposure and tolerability. To accommodate this, delivery devices need to be easily configurable.4
While OBIs often involve longer development timelines than standard autoinjectors, they offer significant advantages once established, including the ability to handle a broad range of formulations and easier configuration across different dosing regimens. By contrast, large-volume autoinjectors have more limited formulation flexibility and may require substantial modifications to support changes in injected volume, which is especially true of spring-powered systems.
One key opportunity identified by The Subcutaneous Drug Development and Delivery Consortium is the use of bridging strategies informed by pharmacokinetics and pharmacodynamics to guide the transition from IV to SC formulations.4 In this context, in silico device and tissue modelling plays a pivotal role in optimising device selection and predicting performance for a given therapeutic profile, supporting early, informed decision-making ahead of clinical development.
OPTIMISING INJECTION PARAMETERS
For both device types, identifying the optimal balance between injection speed, volume and formulation properties remains a significant challenge. The interplay of device, tissue mechanics and formulation creates a highly complex, multi-dimensional parameter space, while limited clinical data further complicates predictions. Even sophisticated modelling approaches can only partially capture this complexity. Nevertheless, several groups have developed sophisticated models to better understand the mechanics and pharmacokinetics of large-volume SC (LVSC) injections:
- Hou et al (2021)5 used a multiphysics finite-element model treating SC tissue as a poroelastic medium, coupling tissue deformation, interstitial fluid dynamics and lymphatic uptake. Their simulations captured steep local pressures and slow clearance but relied on assumptions of tissue homogeneity and ignored vascular heterogeneity.
- Pepin et al (2023)6 introduced SubQ-Sim, a physiologically based biopharmaceutics model integrating device parameters, depot formation and variability from disease states and “life events”, revealing dynamic links between back-pressure, depot geometry and formulation losses.
- Li et al (2024)7 advanced multi-scale modelling by coupling macroscale skin transport with mesoscale lymphatic uptake, highlighting non-uniform drug distribution, the dominant role of initial lymphatics and the critical influence of binding interactions.
Despite these advances, variability between patients in tissue permeability, extracellular matrix properties and lymphatic function complicates defining universal injection parameters.
ACCOUNTING FOR POPULATION HETEROGENEITY
Predictive modelling can help anticipate thresholds where tissue stress, pain, or suboptimal drug distribution may occur. Computational models and practical testing strategies can be used to bring clarity to the complex problem of tissue response during large-volume injections. By simulating tissue deformation, interstitial fluid dynamics and lymphatic uptake, these models identify regions of elevated pressure, potential depot formation and back-pressure that influence patient tolerability and pharmacokinetics.
“INTEGRATING MODELS WITH EXPERIMENTAL DATA CREATES AN ITERATIVE FEEDBACK LOOP IN WHICH SIMULATIONS INFORM EXPERIMENTAL DESIGN AND EXPERIMENTAL RESULTS REFINE THE MODELS.”
However, models alone cannot capture tissue heterogeneity or individual variability. Experimental validation using ex vivo tissue analogues, synthetic gels, or animal models can provide real-world insight under varying injection volumes, viscosities, needle geometries and flow rates. Integrating models with experimental data creates an iterative feedback loop in which simulations inform experimental design and experimental results refine the models. This approach can guide needle and delivery mechanism specifications and define appropriate windows for injection rates, optimising devices to be robust, physiologically appropriate and capable of delivering large-volume SC injections safely and effectively.
RISK
Risk considerations differ between OBIs and large-volume autoinjectors. OBIs involve slow, prolonged deliveries, which introduce the potential for adhesive failure and incomplete dosing. Electronic or connected OBIs can help mitigate these risks through adherence monitoring, guided setup and automated alerts, but these features also add complexity for the user and extend the path to regulatory compliance, lengthening development timelines.
Large-volume autoinjectors rely on high-power mechanisms to deliver the formulation within an appropriate timeframe. Rapid activation of these mechanisms can cause component or container failure, while sustained mechanical stress may result in creep, potentially triggering premature activation. As described previously, they also require longer delivery times. Coupled with often larger and heavier form-factors, this can create additional challenges related to user dexterity and comfort.
Overall, selecting a delivery device for large-volume formulations requires careful balancing of multiple factors. OBIs provide slow, controlled delivery with reduced tissue stress but come with added device complexity, while high-powered autoinjectors offer rapid delivery but introduce mechanical risks and physical limitations. Collaborating with partners who possess in-depth expertise in human factors, device physics and development can help address these challenges early in the development programme, improving both device performance and patient outcomes.
ENABLING ADVANCES IN TECHNOLOGY
Advances in materials, adjuvants and digital technologies are critical enablers for large-volume SC delivery. Adjuvants such as hyaluronidase are being explored to improve tissue permeability, though their effectiveness for pain reduction and effectiveness for the delivery of extremely viscous formulations maybe limited (Figure 3).4
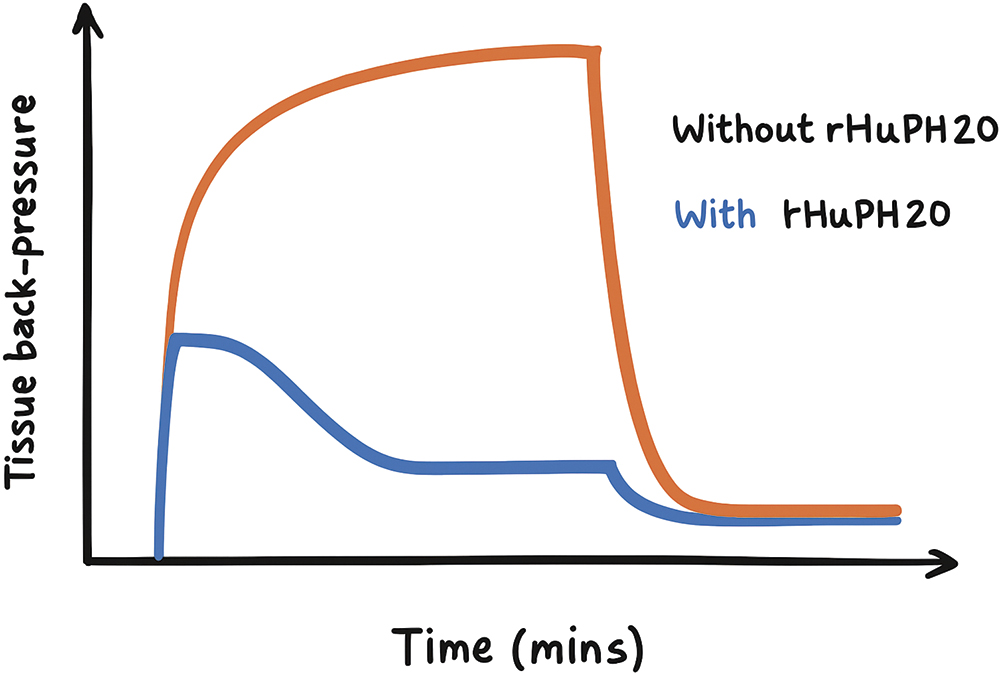
Figure 3: The effect of hyaluronidase adjuvant on the tissue back-pressure experienced during a 10 mL, 7 cP injection over several minutes. Note that the effect becomes apparent after a certain timescale during delivery and that a plateau is reached during the delivery. Reproduced from Pepin et al (2023).
Adhesive systems have evolved rapidly, with sweat-adaptive, light, hydrogel films providing strong, conformal adhesion while maintaining comfort and breathability.8,9 These hydrogels dynamically respond to moisture, reducing skin irritation during extended wear and ensuring secure device placement.
Figure 4: The growing adoption of wearables in both consumer and diabetes care is driving advancements in connectivity and adhesive technologies, enhancing usability, comfort and extending wear time.
Connected OBIs integrate companion apps and cloud connectivity for remote monitoring, guided setup and automated alerts. While supporting adherence, patient safety and clinical oversight, these systems introduce regulatory considerations for Software as a Medical Device, including clinical validation, cybersecurity safeguards and lifecycle management.
Device performance is influenced by drive mechanisms, needle geometry and material selection, which interact with formulation viscosity and injection volume. Optimising these parameters is essential to maintain reliable delivery. Predictive modelling and bench testing can help guide adhesive selection, device ergonomics and flow control, ensuring safe and effective administration.
“AS PATIENT POPULATIONS BECOME INCREASINGLY TECH-SAVVY, ADOPTION OF CONNECTED OBIs IS EXPECTED TO ACCELERATE, OFFERING BOTH CLINICAL ADVANTAGES AND PRACTICAL FEASIBILITY FOR LARGE-VOLUME SC THERAPIES.”
As patient populations become increasingly tech-savvy, adoption of connected OBIs is expected to accelerate, offering both clinical advantages and practical feasibility for large-volume SC therapies (Figure 4).
ADOPTION IN LARGE-VOLUME APPLICATIONS
Current adoption trends illustrate the impact of these considerations on the device market. OBIs have been on the market longer and benefit from an early adoption advantage, whereas truly large-volume autoinjectors (≥5 mL) are more recent innovations and have only just begun to see extensive development. The technological advantages of OBIs, combined with growing industry and patient acceptance, position them as the likely dominant platform for large-volume SC therapies.
Autoinjectors: Large-Volume Devices in Development
The following are the current key players in the large-volume autoinjector field:
- Maggie® 5.0 mL (syringe-based): SHL Medical has partnered with Grand River Aseptic Manufacturing to offer fill-finish services for this ready-to-use cartriQ 5 mL cartridge, co-developed with SCHOTT Pharma.
- YpsoMate 5.5 mL (syringe-based): This device from Ypsomed features an integrated prefilled syringe developed in collaboration with SCHOTT Pharma.
- Bios platform (syringe-based): SMC Ltd is among the few vendors to report a development agreement (October 2024) with a biopharma partner specialising in genetic rare diseases. The device is capable of delivering up to 5 mL of extremely high-viscosity fluids. The only other comparable device in this list is the Kaléo Aerio, although information is more limited.
- Aerio™ platform (cartridge-based): A platform by Kaléo (Richmond, VI, US), the maturity of the large-volume variant is unclear; the AerioDuo (up to 20 mL) is currently in preclinical development with an undisclosed pharmaceutical partner. Kaléo also holds approvals for emergency delivery of epinephrine and naloxone via the AerioUno (AUVI-Q). The device is gas-powered and designed for delivering large volumes of highly viscous fluids.
- Windgap Medical’s LVDC platform (cartridge-based): Another gas-powered large-volume, high-viscosity device, Windgap Medical claims delivery of viscosities over 5,000 cP and is conducting feasibility assessments with several pharmaceutical companies.
On-Body Device (OBD) Approvals
While not large-volume, these devices are relevant to the wearables market and represent a milestone in wearable adoption, reflecting growing understanding of usability and validation of core technologies.
- Onpro: Derived from Omnipod technology by Insulet (Acton, MA, US), this OBI delivers Amgen’s Neulasta (pegfilgrastim).
- Coherus (Redwood City, CA, US) UDENYCA OBI: Based on LTS‘s Sorrel platform, this biosimilar to Neulasta was FDA-approved in 2024 for same-day wearable delivery of pegfilgrastim-cbqv.
- SQ Innovation (Zug, Switzerland) Lasix ONYU: Uses a Gerresheimer OBI (up to 3 mL) and received tentative FDA approval in 2024. Commercial availability is expected once an existing product’s exclusivity expires later in the year.
Large-Volume OBI Approvals
Several devices delivering higher volumes have achieved regulatory success:
- Enable Injections‘enFuse®: Received its first US combination product approval in 2023 for Apellis’ (Waltham, MA, US) EMPAVELI (pegcetacoplan), a twice-weekly 20 mL injection. Regulatory approvals expanded in 2025 in the EU, UK and Brazil, with ongoing clinical studies involving Roche (Basel, Switzerland), Sanofi (Paris, France), Apellis Pharma, UCB (Brussels, Belgium), Viridian (Waltham, MA, US), Serina Therapeutics (Huntsville, AL, US) and Sobi (Stockholm, Sweden).
- West Pharmaceutical Services‘ SmartDose: First combination product approval came in 2016 with Amgen’s Pushtronex system for Repatha (evolocumab). After withdrawal, West entered a development agreement in 2019 with scPharmaceuticals (just acquired by MannKind Corporation) for SmartDose 10 mL delivery of FUROSCIX, gaining FDA approval in 2022.
Large-Volume OBI Clinical Programmes
OBIs are increasingly explored for high-dose monoclonal antibody delivery:
- Sanofi’s isatuximab (Sarclisa): SC program using Enable Injections’ enFuse® reported Phase 3 IRAKLIA results in 2025, demonstrating non-inferiority versus IV delivery.4
- BD Libertas system: Available in 2–5 mL and 5–10 mL configurations; entered its first pharma-sponsored clinical trial in 2025.
- Stevanato Group’s Vertiva: Available in 3 mL and 10 mL formats, with new human-factors and sustainability data recently presented.
- Ypsomed’s YpsoDose: Supports injections up to 10 mL for high-viscosity formulations; collaborations with SCHOTT (RTU FIOLAX cartridges) and CDMO ten23 health support fill-finish and assembly.
- LTS (Sorrel) OBIs: Following LTS’s 2023 acquisition of Sorrel, the low-volume UDENYCA OBI received FDA approval at the end of 2024; the 5–20+ mL device is in development with multiple pharmaceutical partners.
- Gerresheimer Gx SensAir OBI concept: Integrates digital health features by Aptar Pharma for adherence monitoring; expanded ready-to-fill syringe and cartridge production which supports SensAir and related programs. Regulators are cautiously supportive, closely monitoring safety and usability.
WHAT IS THE PROGNOSIS?
The evolution of large-volume SC drug delivery reflects a complex interplay between device performance, biological constraints and patient preference. Traditional autoinjectors remain simple and familiar, but their advantages diminish as injection volumes and viscosities increase. High-power requirements introduce mechanical risks and rapid injections can cause pain.
OBIs, increasingly familiar to both industry and users, along with advances in adhesive and sensing technologies, offer a practical solution for delivering high-volume biologics safely and consistently. Extended-duration, hands-free administration reduces tissue stress and interstitial pressure, improving tolerability.
Looking ahead, OBIs are well positioned to play a leading role in the delivery of large-volume biologics, but the choice between autoinjectors and OBIs is not straightforward. Patient preferences vary – some prioritise the speed and discretion of autoinjectors, while others accept longer wear times for greater comfort and dosing flexibility. Safety, cost, regulatory requirements and development timelines also influence adoption, with each stakeholder group bringing distinct priorities. The future of delivery lies in tailored device strategies optimised for specific therapies, clinical contexts and patient needs.
Engaging a multidisciplinary CDMO early in development can reduce costs, de-risk technical challenges and strengthen supply chains. With expertise in device design, human factors, risk management and design-for-manufacture-and-assembly for later scale-up.
[Publisher’s Note: Many of the OBIs discussed here above were featured in the recently published Wearable Injectors issue of ONdrugDelivery, Issue 176, Sep 2025. Read the full issue here.]
REFERENCES
- Ziklstra E et al, “Impact of Injection Speed, Volume, and Site on Pain Sensation”. J Diabetes Sci Technol, 2017, 12(1), pp 163–168.
- Schwarzenbach F et al, “Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance”. Med Devices (Auckl), 2015, p 473.
- Doughty DV et al, “Understanding subcutaneous tissue pressure for engineering injection devices for Large-Volume protein Delivery”. J Pharm Sci. 2016, 105(7), pp 2105–2113.
- Bruin G et al, “An industry perspective on clinical development and regulatory strategies for subcutaneously administered high-dose biologics”. J Control Release, 2025, 386, p 114156.
- Hou P et al, “Multiphysics modelling and simulation of subcutaneous injection and absorption of biotherapeutics: sensitivity analysis”. Pharm Res, 2021, 8(6), pp 1011–1030.
- Pepin XJH et al, “SubQ-Sim: A Subcutaneous Physiologically Based Biopharmaceutics Model. Part 1: The Injection and System Parameters”. Pharm Res, 2023, 40(9), pp 2195–2214.
- Li C et al, “A multi-scale numerical study of monoclonal antibodies uptake by initial lymphatics after subcutaneous injection”. Int J Pharm, 2024, 661, art 124419.
- Wang G et al, “Biomimetic breathable nanofiber electronic skins with temperature-controlled self-adhesive and directional moisture-wicking properties for bifunctional pressure and non-contact sensing”. Nano Energy, 128, art 109779.
- Zhang et al, “Sweat-enhanced adhesive hydrogel enables interfacial exchange coupling for wearable strain sensor”. Chemical Engineering Journal, 2024, 495, art 153385.

