Citation: Bedford T, “Meeting Emerging Stakeholder Needs with the Subcuject Wearable Bolus Injector”. ONdrugDelivery, Issue 124 (Sep 2021), pp 46–50.
Tony Bedford looks at the emerging market for on-body injectors coupled with stakeholder needs and introduces the Subcuject wearable bolus injector, which is designed to accommodate these requirements.
“The prospect for OBIs remains promising, driven by new biologic drugs, the reformulation of intravenous drugs for subcutaneous routes of administration and the continued push for more widespread self-administration.”
In ONdrugDelivery’s previous issue on Wearable Injectors (Issue 111, Sep 2020), our article discussed emerging trends that could shape the market for on-body injectors (OBIs). At that time, these were the potential erosion of OBI market share by autoinjectors at lower volumes, the switching of already marketed intravenous drugs to subcutaneous formulations, the long-term impact of covid-19 and a tendency towards prefilled device configurations.
The prospect for OBIs remains promising, driven by new biologic drugs, the reformulation of intravenous drugs for subcutaneous routes of administration and the continued push for more widespread self-administration. Stakeholders are starting to demand simpler, more cost effective devices and, in this article, we will look at some of the evolving stakeholder needs and introduce the Subcuject wearable bolus injector (WBI) designed to meet those needs.
VOLUME-BASED SCENARIOS
Subcutaneous formulation research allows us to assemble a set of volume-based scenarios that can be used to determine the optimal device configuration, including where the drug might be administered and what drives these positions.
To reduce dosing frequency, a new breed of biologic drugs is being formulated for subcutaneous administration. Formulation teams are trying to pack higher concentrations of active ingredients into their product, which increases viscosity and/or results in larger delivery volumes to balance the need for higher concentrations with a product that can be injected without damaging the molecules or causing discomfort for the patient. We can, therefore, expect to see an increase in subcutaneously administered drugs with volumes of 2–5 mL and beyond begin to appear, and it is within this volume bracket that competition between delivery devices will intensify.
Anecdotally, it is feasible for a patient to receive a dose made up of two consecutive injections from 2.25 mL autoinjectors (which are already launched on the market and successfully delivering doses), and we fully expect even larger volume autoinjectors to reach the market. However, questions remain over the practicalities and patient comfort of administering injections in this way – for example, ensuring patients are sufficiently trained to use both autoinjectors (in the case of a pack of two equalling a single dose) rather than mistakenly considering one to be a spare, avoiding under-dosing through wet injections (where the autoinjector is lifted away from the skin prior to completion of delivery) and dealing with the absorption and pain issues caused by repeated injection at the same site or the need to hold the autoinjector still for a longer time period.
Consequently, the 2–5 mL volume range is a good starting point for wearable devices to establish a market position as it provides an alternative to multiple or larger autoinjectors and can offer a slower delivery rate that may be better suited to more viscous and larger volume drugs. Perhaps even better is the next range, 5–10 mL, which is likely to be beyond the reach of larger embodiments of autoinjectors and seems ideal for cost-effective single-use and reusable OBIs alike. With the knowledge that a number of OBIs with different configurations are in development, formulation teams may relax a little instead of striving for the highest possible concentration and the lowest possible volume, particularly in cases where packing the drug into a prefilled syringe or autoinjector is not a realistic target.
The last group is the 10 mL and above range, already populated by “switched” products such as Janssen’s DARZALEX Faspro® (daratumumab and hyaluronidase-fihj) and Roche’s MabThera SC (rituximab), which are currently delivered from syringes as fixed doses of 15 mL and 11 mL, respectively. Both of these were originally (and still are) marketed for the intravenous route of administration and herald the start of an expected rush of “switching”, particularly in the immuno-oncology space – for example, Merck & Co’s top-selling Keytruda® (pembrolizumab) is currently in clinical trials in subcutaneous form. We expect to see drugs such as these being delivered via some form of on-body delivery system in due course.
Figure 1 illustrates these groupings, with the formulation of smaller volume biologics driven by the need for less frequent (and most likely self-) administration, and the reformulation of larger volume products driven by the need for decreasing cost, with significant savings made possible by reducing time in the clinic, hands-on involvement by a healthcare practitioner and, potentially, moving administration away from the clinic altogether.
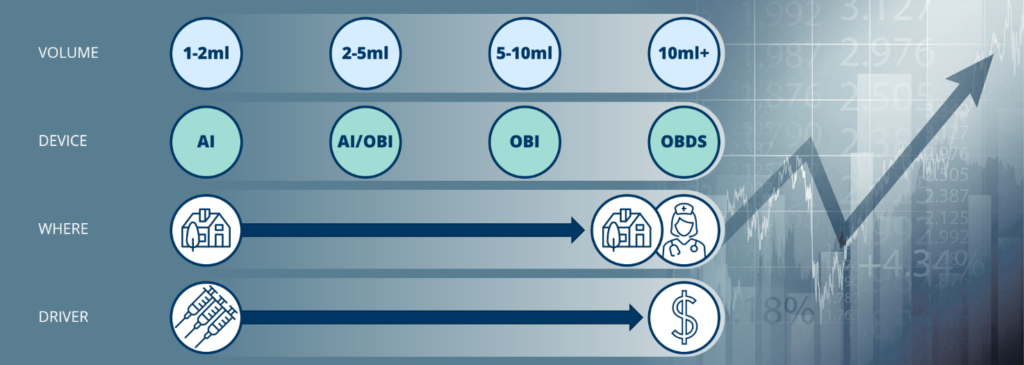
Figure 1: Subcutaneous volume groupings, devices and drivers.
“Perhaps the most influential stakeholder group is the pharmaceutical or biotech company whose drug is contained within the device.”
EVOLVING STANDARDS
Certainly, it appears as though regulators believe that there is potential for a significant increase in the number of OBIs reaching the market and, presumably derived from some of the learnings from devices that are already commercialised, a number of changes are being drafted for the latest update of the ISO 11608 series, which are expected to be published during 2022.1 This is understood to include moving some of the elements specific to ISO 11608-62 to ISO 11608-13 and the addition of fluid lines and paths to ISO 11608-3,4 signalling intent with regard to the arrival of OBIs.
STAKEHOLDER NEEDS
In order to succeed in this emerging market, a number of stakeholder needs must be met. Given the relatively low market penetration of OBIs thus far, we do not yet know for sure if patients will accept these body-worn devices, so that is a good place to start.
Autoinjectors offer a simple, two- or three-step process for delivering a dose; OBIs must compete with this as it is unlikely that patients will be satisfied with anything that increases the burden. Perhaps the need we hear about the most is for devices that are prefilled and preloaded – this minimises the need for user involvement or increased patient burden, but interestingly, none of the wearable devices currently on the market adopt a prefilled and preloaded format. With this configuration, all that is required of the user is to remove the device from the primary packaging, apply the device and activate it.
Patients also need to know the status of the device – Is it working? Has it completed the dose? – and these are issues that are being increasingly scrutinised by the regulators, so high-quality design and user feedback are of paramount importance. The patient needs are likely echoed by healthcare practitioners too. This stakeholder group should be considered as a gatekeeper, or at the very least an influencer, when it comes to the adoption of devices as they reach the market; if choice is available, the option that works reliably and can be trusted in the hands of the patient will emerge as the winner – but also anything that reduces healthcare practitioner burden (e.g. replacing a large volume syringe) should be warmly received.
Perhaps the most influential stakeholder group is the pharmaceutical or biotech company whose drug is contained within the device. With the uptake of drug delivery devices on the increase, drug companies have become increasingly savvy and sensitive to the needs of their customers and, in addition to their own needs, will also look to ensure that those of other stakeholders are being met. Gone are the days of the device and patient comfort being disregarded, if ever that was really the case.
Pharmaceutical and biotech companies need to be certain of the efficacy of any device. Put simply, they have to work reliably and accurately, all the time, without risking the stability of the drug through, for example, shear damage, material compatibility or temperature-related issues.
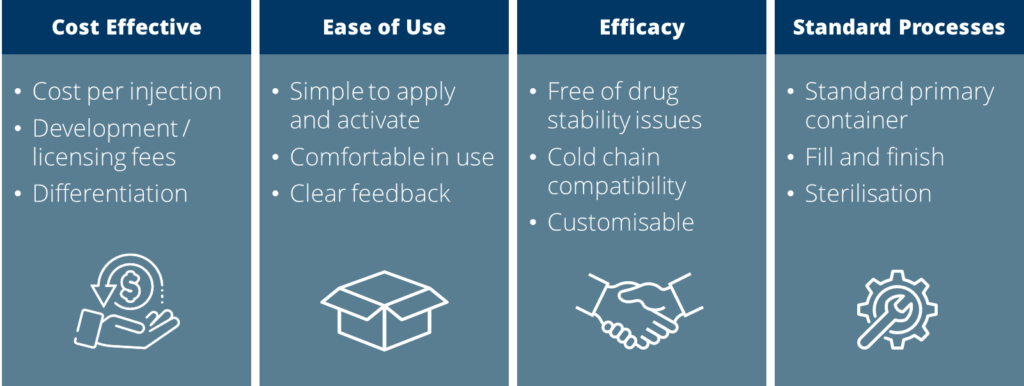
Figure 2: Stakeholder needs for OBIs.
“Combining the elements of larger volume and ease of use together results in a device that readily crosses from self-administration to clinic-based use – easily applied and used by patients and healthcare practitioners alike.”
Add to those needs the requirement for fill and finish (and sterilisation) without disruption or significant investment in new processes, and an overwhelming desire to use trusted primary containers to minimise stability and handling issues, and the blueprint for the specification of any OBI development is already partially drafted. These example needs across stakeholders are summarised in Figure 2.

Figure 3: The Subcuject WBI.
THE SUBCUJECT WEARABLE BOLUS INJECTOR
Phillips-Medisize is collaborating with Subcuject in order to bring the Subcuject WBI to market (Figure 3). The Subcuject WBI has been designed with stakeholder needs in mind and offers a cost-effective, pharma-friendly and patient-friendly solution.
The Subcuject WBI is currently configured to deliver up to 5 mL from a prefilled standard glass cartridge to allow ease of integration into filling lines without deviation from industry-standard stopper or container materials. The design allows for flexibility in terms of delivery volume (the device is a fixed-dose bolus injector) – either by underfilling or by employing a smaller cartridge – and is a scalable platform design, which in-laboratory bench tests have already demonstrated can deliver up to 15 mL from a larger primary container.
It is also preloaded, meeting the needs of the end-user by minimising the steps required to use the device – once removed from the primary packaging, it is a simple three-step “peel, place, press” process to complete the dose (Figure 4).
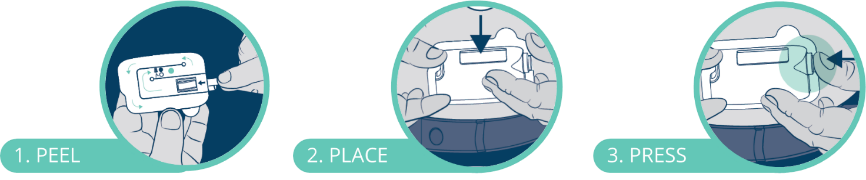
Figure 4: Three-step use of the Subcuject WBI.
With built-in needle insertion and retraction, the user has control at the beginning of the treatment and safety at the end, with feedback to suit the usability requirements.
Furthermore, the device is conveniently small compared with other OBIs and is noiseless during operation, apart from “click” sounds at activation and at end of dose.
Combining the elements of larger volume and ease of use together results in a device that readily crosses from self-administration to clinic-based use – easily applied and used by patients and healthcare practitioners alike. The Subcuject WBI can also be used in scenarios where the patient continues with their daily tasks or is observed during use (for example, some immuno-oncology drugs require patient supervision and availability of resuscitation equipment upon first dose).
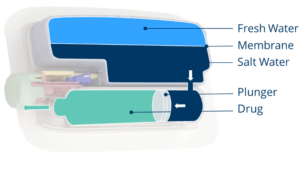
Figure 5: Forward osmosis delivery of drugs from a standard cartridge.
A COST-EFFICIENT, NATURE-INSPIRED SOLUTION
What is really compelling about this technology is the novel drive system, which is free of batteries, electronics and motors, maintaining simplicity and facilitates a much lower cost of goods than electromechanical devices. Whilst sustainability credentials are a challenge for any single-use disposable device, the Subcuject WBI offers an alternative to throwing away a greater number of (smaller volume) devices by way of its larger payload and/or reduced frequency of use, while also eliminating the need for the disposal of battery chemicals and numerous metallic components.
By using forward osmosis, the device creates a hydraulic pressure internally once it has been activated by allowing salt and freshwater to mix (Figure 5). The hydraulics replace the need for lead screws, plunger rods or other mechanical solutions and drive the stopper along, expelling the drug. This is all done naturally, requiring just the simple press of a button to start the process. This provides a unique solution free of superfluous functionality and complexity.
ACCURATE AND VERSATILE
As previously noted, the expected new breed of biologic drugs is likely to see increasing viscosities and these pose no issue for the Subcuject WBI. A characteristic of osmosis is that it will build up a higher pressure when meeting a counterforce, and initial tests have successfully and repeatedly delivered liquids up to 100 cP via a 27G needle, the trade-off being a slight decrease in flow-rate (at 1 cP the Subcuject WBI delivers at a rate of approximately 1 mL per minute), with a high degree of dose delivery accuracy. This makes for an incredibly versatile platform.
With 5 mL demonstration devices expected to be available during the last quarter of 2021, Phillips-Medisize and Subcuject are ready for engagement into drug-specific feasibility and development programmes with pharmaceutical and biotech companies.
ON-BODY INJECTION IS ON THE WAY
Ever since Dr Arnold Kadish’s invention of the first backpack-sized wearable insulin pump in 1963, we have been on an exciting path towards wearable drug delivery for all kinds of therapeutic areas. To those who have reached the market already – hearty congratulations and kudos. To those who are on their way – to paraphrase what we said a year ago, it feels like we are accelerating down the road on an exciting journey.
REFERENCES
- Augustyn S, “ISO 11608: All change for injector standards”. Cambridge Design Partnership blog, Apr 1, 2021.
- ISO/FDIS 11608-6 “Needle-based injection systems for medical use – Requirements and test methods – Part 6: On-body delivery systems”. ISO, under development.
- ISO/FDIS 11608-1 “Needle-based injection systems for medical use – Requirements and test methods – Part 1: Needle-based injection systems”. ISO, published 2014.
- ISO/FDIS 11608-3 “Needle-based injection systems for medical use – Requirements and test methods – Part 3: Containers and integrated fluid paths”. ISO, under development.
- Kesavadev J et al, “Evolution of Insulin Delivery Devices: From Syringes, Pens, and Pumps to DIY Artificial Pancreas”. Diabetes Ther, 2020, Vol 11(6), pp 1251–1269.

