To Issue 178
Citation: Holden T, “Smarter for Budgets & Kinder to the Planet: Ecosafe® Safety Syringe Platform”. ONdrugDelivery, Issue 178 (Oct 2025), pp 36–40.
Tim Holden describes the key objectives behind Owen Mumford’s 1 mL safety syringe injection platform and how the company sought to provide a single platform for all patients (able, less dexterous and needle-phobic) while minimising cost, waste and carbon footprint impact.
All healthcare providers (HCPs) want as many patients with chronic conditions as possible to adhere to, tolerate and successfully self-administer their injection. To meet this need, Owen Mumford designed its EcoSafe® safety syringe to be easy to use, safe (passive needle shielding), cost effective and low waste (5 g disposable weight). For around four in every five patients, safety syringes enable safe, independent self-administration. But what about those who are needle-phobic or who do not have the manual dexterity to self-administer?
Sensitive Patient Populations
Many studies show that needle-phobia prevents some patients from successfully self-administering.1 Sheer physical, emotional and/or cognitive ability to self-administer is also the subject of multiple studies.2 Most analysts regard the dexterity issue as a particularly important one, affecting a significant proportion of patients and escalating with age, once again making it imperative for a drug delivery device (in the form of an autoinjector) to make the administration process as simple and easy as possible.
This was the challenge faced by the Owen Mumford development team. How could the EcoSafe safety syringe be extended to cover the needs of all patients – the able, the phobic and the less dexterous? And how could that be achieved without pushing up costs or generating unsustainable plastic waste and carbon emissions? The challenge was on for EcoSafe.
WHY IS SUCCESSFUL SELF-ADMINISTRATION FOR ALL PATIENTS SO IMPORTANT?
Drug delivery systems that enable successful self-administration for all patients form the ideal solution for HCPs. Why? Well, there are three reasons:
- Successful Self-Administration Typically Delivers Substantial Healthcare Cost Savings: All round the world, healthcare payers are urgently seeking ways of minimising spiralling costs (resulting from ageing populations and new therapies). Patient self-administration helps healthcare systems cope better with staff shortages. Staff time spent administering drugs to patients unnecessarily – where this can be avoided through self-administration – is hugely wasteful and expensive.
- A Single Drug Delivery Platform Covering All Patient Needs Has Multiple Patient and Procurement Benefits: HCPs are looking to simplify procurement. This reduces administrative time and cost, simplifies drug administration for HCPs and provides a single consistent platform for patient training, comfort and experience.
- Successful Self-Administration at Home Has a Hugely Positive Impact on Waste and Carbon Footprint: If self-administration fails – because the patient is insufficiently dexterous to handle a standard drug delivery device (e.g. a safety syringe), or they are needle-phobic or have cognitive (mental) issues – then they either have to be visited by a district nurse or they need to go (or be taken) into hospital. The carbon footprint of this unnecessary activity – for instance, road transport – is so great that it makes CO2 emission comparisons between drug delivery devices pale into relative insignificance (Figure 1).
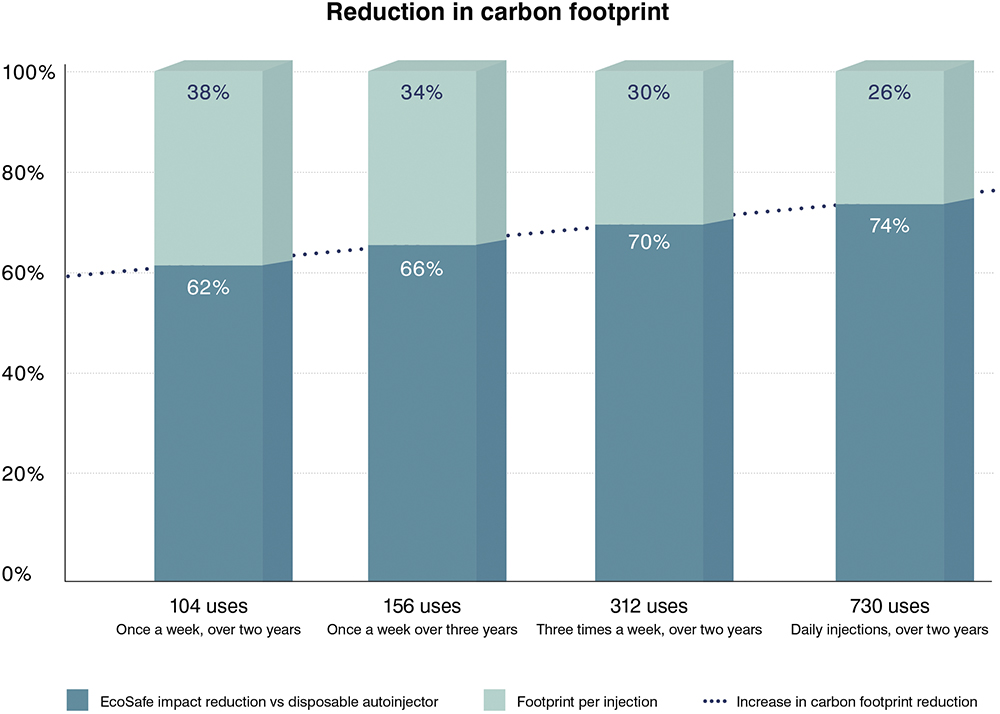
Figure 1: Carbon footprint reduction per injection – reusable EcoSafe autoinjector versus a disposable autoinjector.
A review of injectable therapies concluded that home injection was associated with economic savings compared with healthcare-setting administration.3 Studies consistently show that when patients with multiple sclerosis can self-inject subcutaneous biologics, the total annual cost per patient drops significantly (>40%) compared with hospital-based administration.4 A clinical paper on asthma biologic administration found that home administration was typically significantly more cost effective.5 An Australian study found that home-based self-administration of immunoglobulin delivered savings of over 80% over hospital administration.6
“WITH THE ECOSAFE PLATFORM, OWEN MUMFORD AIMED TO CREATE AN OPTIMAL SOLUTION FOR ALL PARTIES – PATIENTS, PHARMA COMPANIES AND HEALTHCARE PROFESSIONALS AND SYSTEMS.”
CONCEPTUAL BREAKTHROUGH – A “COMPANION” REUSABLE AUTOINJECTOR
With the EcoSafe platform, Owen Mumford aimed to create an optimal solution for all parties – patients, pharma companies and healthcare professionals and systems.
The conceptual breakthrough was to regard the EcoSafe 1 mL safety syringe as the “cartridge” component of the EcoSafe reusable autoinjector. The optional “companion” – a reusable autoinjector – becomes a wrap-around solution when combined with the safety syringe for those sensitive patient groups.
For the pharma company, engineering, product development and combination product assembly/filling is made far more efficient through a single product family concept. Prefilled safety syringes also act as the autoinjector’s cartridge – one single, simple filling line with no variations for sensitive patients. No longer needing to offer different drug delivery products across a single therapy. Production economies of scale come into play. And the commercial proposition for healthcare customers is simple, integrated and unified – no gaps, no complications.
THE ECOSAFE STORY
Owen Mumford’s development team set out to achieve three key goals that would offer benefits to patients, HCPs and their pharmaceutical suppliers alike, designing a single 1 mL safety syringe platform that:
- Encourages adherence through inclusive design – serving the able, the non-dexterous and the phobic alike
- Delivers significantly lower cost-per-dose for sensitive patients by removing HCP costs associated with dose administration
- Minimises carbon footprint across the range of dosage scenarios (validated through an independent lifecycle analysis (LCA).
The inclusivity solution has already been described – extending the safety syringe platform with an optional companion autoinjector. But how did the team also manage to deliver minimised cost-per-dose, along with reduction in plastic waste and carbon footprint?
HCPs are looking for reliable solutions that improve patients’ lives while minimising cost and footprint. As such, there is a growing trend towards devices with a reusable element – but the savings achieved will depend on frequency of use.
It is considerably harder to develop a reusable platform device that is optimised for both cost and sustainability than a single-use device with a specific purpose.7 Ultimately, the success of reusable platforms depends on whether they deliver a safe, convenient and trustworthy experience for patients while reducing waste.
As well as covering the full gamut of patient self-administration needs, including the less dexterous and the needle-phobic, the EcoSafe safety syringe plus companion reusable autoinjector was also designed to minimise environmental footprint by offering a sustainable solution.
Sustainability Imperatives
Reducing carbon footprint is now a constant expectation – from patients, from HCPs and from pharma companies. In the US, the Decarbonisation and Resilience Initiative of the Centers for Medicare & Medicaid Services is currently sparking intense debate.8 In Europe, mandatory environmental standards are being developed.9 And across Asia, various sustainability initiatives are already in progress.10
To support the new EcoSafe reusable autoinjector with robust data, Owen Mumford commissioned an independent LCA to examine:
- What is the carbon footprint per injection of the combined EcoSafe® safety syringe and reusable EcoSafe® autoinjector?
- How does frequency of use affect the impact of the reusable autoinjector?
Firstly, the carbon footprint per injection is under 0.1 kg CO2e. This is the case even for just 100 uses, and it marks a new milestone in absolute carbon footprint reduction.
This carbon emissions value is the reusable autoinjector’s contribution plus the impact of the disposable EcoSafe safety syringe. This level of associated emissions appears to be far less than published emissions data on other reusable autoinjectors on the market. Moreover, other reusable autoinjectors do not incorporate a safety syringe to play the part of a standard “cartridge”.
Reducing Plastic Waste
Other than substituting materials that are truly less harmful than fossil fuel-derived plastics,11 one strategy to reduce materials impact is to minimise the amount of plastic used. Coupled with a simple design and a minimal number of parts (think of the EcoSafe syringe’s springless design), this strategy aids end-of-life recycling and disposal.
A disposable autoinjector typically weighs around 25 g. One hundred uses produces over 2 kg of waste. A reusable solution, which incorporates the extremely light EcoSafe safety syringe (just 5 g), can considerably reduce those waste levels.
Pharma companies and healthcare users therefore have a vested interest in exploring reusable companion autoinjector solutions for their phobic or less dexterous patients. The graph in Figure 2 illustrates just how substantial the waste reduction can be versus using disposable autoinjector options. This is becoming increasingly important as healthcare organisations embed mandatory waste-reduction programmes in their ongoing operational policies.12
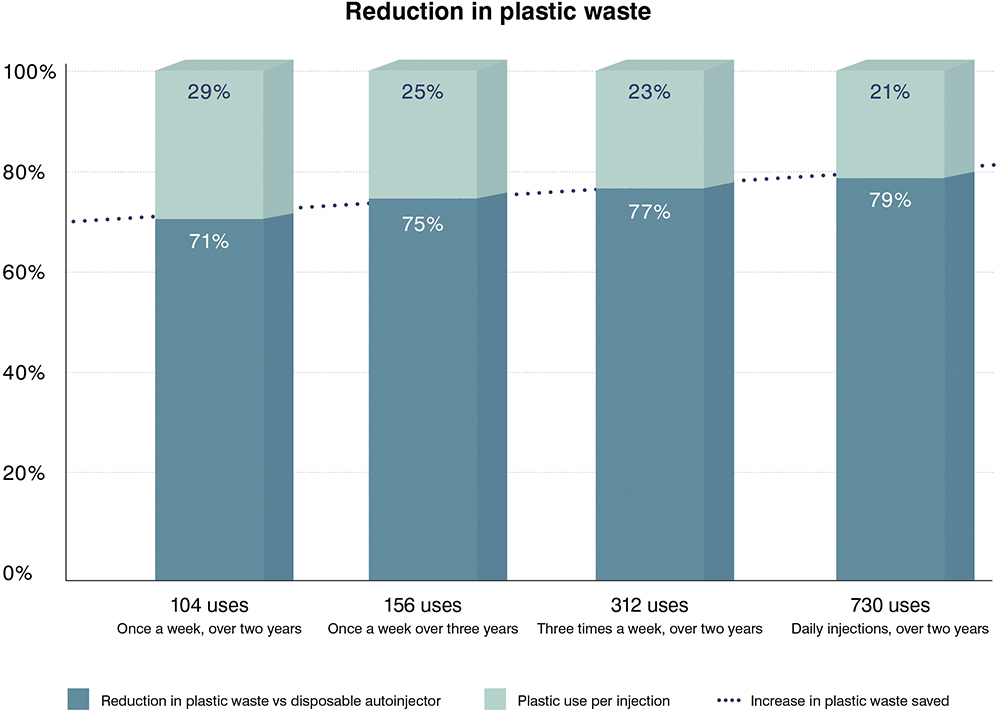
Figure 2: Plastic reduction with EcoSafe.
Why Assess Carbon Footprint Per Injection?
The functional unit for this LCA study was: “The injecting of a single dose of pharmaceutical using a 1 mL EcoSafe safety syringe with minimum and maximum fill volumes of 0.1 mL, with an EcoSafe reusable autoinjector.”
Assessing the impact of a drug delivery device across its lifecycle must consider the number of uses – but these vary. Determining footprint per injection offers a starting point for a number of calculations – whether a device is used once or hundreds of times.
CONCLUSIONS
Clearly, a sustainability assessment is not a once-and-done activity. Owen Mumford has developed its own internal, bespoke lifecycle-based eco-design tool in collaboration with a world-leading LCA consultancy, PRé Sustainability (Amersfoort, the Netherlands). The tool allows the user to autonomously build various scenarios, assess different product concepts and configurations – including material choices, component weights, packaging, efficiency, transportation, material supply and manufacturing location and various end-of-life scenarios.
“THE SAFETY SYRINGE AND COMPANION AUTOINJECTOR ARE REGARDED AS PART OF THE SAME PRODUCT FAMILY, WITH THE 1 ML SAFETY SYRINGE ACTING AS THE “CARTRIDGE” FOR THE AUTOINJECTOR. THE RESULT IS ONE PRODUCT PLATFORM COVERING ALL PATIENT GROUPS – ABLE AND SENSITIVE.”
In the meantime, the Owen Mumford approach has managed to reconcile apparently competing factors. The safety syringe and companion autoinjector are regarded as part of the same product family, with the 1 mL safety syringe acting as the “cartridge” for the autoinjector. The result is one product platform covering all patient groups – able and sensitive. This minimises expensive self-administration “fails” that result in an HCP call or a hospital visit.
Reusability is engineered for a product lifetime of over a thousand uses. The result is achieving cost-control by diluting across multiple uses, meaning better value and functionality for all patients without the escalation in cost associated with disposable options. Carbon emissions and plastic waste are also minimised, and are only marginally more than the use of a safety syringe on its own. This contributes positively to sustainability goals for HCPs and pharmaceuticals companies alike.
EcoSafe set out to fulfil all these objectives. The data shown in this article illustrate how this was achieved and the extent of that success (Box 1).
BOX 1: ECOSAFE® SAFETY SYRINGE PLATFORM – CONFIDENCE IN EVERY DOSE
EcoSafe Safety Syringe
An eco-friendly, spring-free safety syringe range. The only safety syringe that helps needle-phobic patients’ confidence, offering a reusable autoinjector*:
- Intuitive: A familiar passive injection technique
- User-Friendly: Full visibility of syringe barrel for user assurance
- Cost Effective: Simplified assembly, lower manufacturing costs and extended shelf life of three years
- Spring-Free: Lack of spring prevents accidental activation during transit
- Secure: Integrated plunger rod stays sealed in packaging, minimising risks of drug spillage, contamination and container closure integrity issues
EcoSafe autoinjector
A reusable, mechanical companion autoinjector for the EcoSafe 1 mL safety syringe:
- User-Friendly: Designed to offer a calmer, more confident injection experience, making it ideal for improving adherence in sensitive patient populations
- Cost Effective: Lower cost per dose than single-use autoinjector and therefore highly economical for frequently dosed therapies
- Sustainable: EcoSafe safety syringe is the only disposable plastic part and weighs only 5 g – significantly less than cassette-based systems
- Simplified Design: Mechanical drug delivery is not dependent on battery function
- Optional Connectivity: Available with connectivity, if required; lifetime battery and internal memory with no charging required and no need for docking stations.
* 1 mL safety syringe version only
REFERENCES
- Wibisono AH et al, “Fear of injections among people with type 2 diabetes: Overview of the problem”. J Diabetes Nurs, 2017, Vol 21(3), pp 91–95.
- Toschi E, Munshi MN, “Benefits and challenges of diabetes technology use in older adults”. Endocrin Metab Clin North Am, 2020, Vol 49(1), pp 57–67.
- Boguszewski CL et al, “Evaluating home injection compared with healthcare-setting injection of somatostatin analogs: a systematic literature review”. Endocrine, 2023, Vol 79(3), pp 527–536.
- Shrestha S, Simonson D, Zagel A, “A Pilot Study of Ocrelizumab Infusion Reaction Rates Among Multiple Sclerosis Patients Treated in a Hospital and 2 Outpatient Sites of Care”. Infusion Journal, 2022, Vol 1(3), pp 10–17.
- Shaker M et al, “Estimation of Health and Economic Benefits of Clinic Versus Home Administration of Omalizumab and Mepolizumab”. J All Clin Immunol Pract, 2019, Vol 8(10).
- Ritchie B et al, “Economic impact of self-administered subcutaneous versus clinic-administered intravenous immunoglobulin G therapy in Alberta, Canada: a population-based cohort study”. Allergy, Asthma Clin Immunol, 2022, Vol 18(10).
- Willoughby A, Tank P-S, “Reusable Versus Single-Use Devices: Trade-offs in Improving Sustainability”. ONdrugDelivery, Issue 159 (Apr/May 2024), pp 24–27.
- Southwick R, “Feds want hospitals, health providers to provide data on carbon emissions”. Chief Healthcare Executive, Apr 2024.
- Gerecke G, Tziambazis E, Hadfield M, “Decarbonising Healthcare: How a Competitive Medical Technology Industry Can Contribute”. MedTech Europe, 2025.
- Bhaskar HC, “Driving Net Zero Healthcare in Asia and beyond”. BioSpectrum Asia Edition, Apr 2024.
- Cho R, “The Truth About Bioplastics”. Columbia Climate School, Dec 2017.
- “NHS clinical waste strategy”. UK NHS, Mar 2023.

