To Issue 178
Citation: Stark S, Schaerer-Lickova S, “The Compass for Innovation: Regulatory Affairs at Ypsomed”. ONdrugDelivery, Issue 178 (Oct 2025), pp 10–15.
Stefanie Stark and Sandra Schaerer-Lickova highlight how regulatory strategy enables ideas to become solutions, supports development through the product lifecycle and helps ensure that patients worldwide obtain safe and timely access.
If you pick up an Ypsomed injection pen or autoinjector, the first things you notice are likely the technology, design or ease of use. These are the tangible outcomes of Ypsomed’s purpose – making self-care simpler and easier. What is less apparent, but equally essential, is the framework that allows such innovations to become reality. Every new idea must navigate a complex landscape of global regulatory requirements before it can safely reach patients. As such, regulatory affairs plays an indispensable role in enabling and supporting innovation.
Regulatory affairs is the part of drug delivery device development that rarely makes headlines. However, it is critical for ensuring that promising concepts don’t remain sketches but develop into trusted solutions that meet the highest standards of safety and performance. If innovation is the visible face of delivery device development, regulatory affairs is the compass that keeps it on course.
“THE GROWTH OF INNOVATIVE BIOLOGICS, FAST-EMERGING BIOSIMILARS AND THE RAPID PENETRATION OF GLP-1S INTO NEW TREATMENTS ARE JUST A FEW EXAMPLES OF HOW THERAPEUTIC AREAS, MOLECULES AND PATIENT NEEDS ARE ALL EVOLVING.”
YPSOMED’S CORE VALUES AND THEIR REGULATORY RELEVANCE
In today’s drug delivery environment, the pace and complexity of development are ever increasing. The growth of innovative biologics, fast-emerging biosimilars and the rapid penetration of glucagon-like peptide-1 agonists (GLP-1s) into new treatments are just a few examples of how therapeutic areas, molecules and patient needs are all evolving.
Figure 1: Ypsomed’s core values and their regulatory relevance.
There is a rising demand for devices that enable outpatient and, in particular, at-home care. This is partly driven by more engaged patients who expect user-friendly, sometimes connected solutions and a seamless user experience with their medication. Simultaneously, regulatory priorities are evolving in step with geopolitical shifts, creating a growing trend toward mandatory localisation, more rigorous documentation and enhanced supply chain visibility across different markets. In this rapidly evolving environment, Ypsomed’s four strategic pillars serve as guiding principles (Figure 1):
- Commitment to innovation
- Standardised yet highly adaptable device platforms
- Operational excellence
- A deep sense of responsibility.
“THIS “REGULATORY RADAR” ENABLES THE COMPANY TO CONTINUOUSLY MONITOR GLOBAL FRAMEWORKS, ANTICIPATE UPCOMING CHANGES AND ASSESS THEIR IMPACT ON ITS PRODUCT PORTFOLIO.”
Over the past decades, these values have enabled Ypsomed to establish a mature and proactive approach to regulatory partnership. Rather than simply reacting to new health authority requirements or waiting for the new guidelines to emerge, Ypsomed integrates regulatory expertise as one of several guiding factors throughout development, from innovation to early platform design and throughout the product lifecycle.
This philosophy ensures that innovation is consistently aligned with both regulatory expectations and the evolving needs of patients and pharma partners. Innovation often starts with a new idea – as part of this, Ypsomed considers regulatory feasibility early on, alongside user needs and technical design, asking “Can this idea survive the scrutiny of global regulators?”
PLATFORMS SUPPORTED BY REGULATORY INSIGHT
Figure 3: Regulatory intelligence as a differentiator.
Ypsomed’s platform portfolio – ranging from injection pens to autoinjectors and patch injectors (Figure 2) – embodies both technical innovation and regulatory strength, enabling pharma partners to advance new therapies with greater speed and reduced risk. To stay ahead in a rapidly evolving healthcare environment, Ypsomed invests dedicated resources into regulatory intelligence (Figure 3). This “regulatory radar” enables the company to continuously monitor global frameworks, anticipate upcoming changes and assess their impact on its product portfolio.
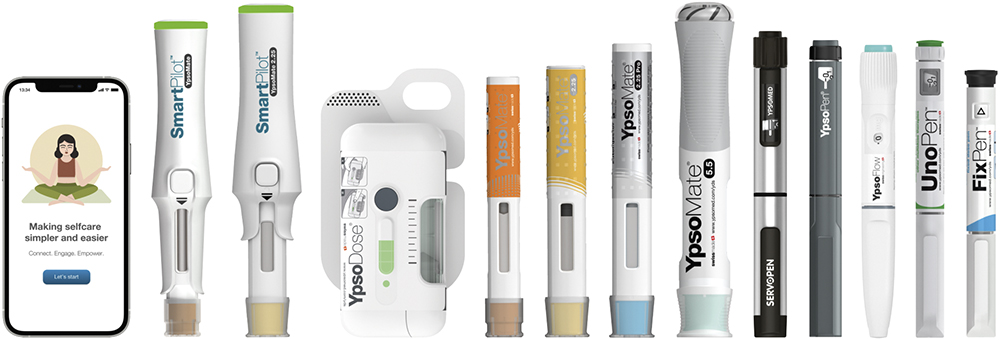
Figure 2: Ypsomed’s comprehensive portfolio including pens, autoinjectors, patch injectors and digital health.
To remain aligned with evolving regulatory expectations, Ypsomed follows a structured, cross-functional process focused on the following practices:
- Proactive Engagement: Ypsomed continuously monitors international regulatory trends, with contributions made to public consultations on draft laws and standards when opportunities arise.
- Cross-Industry Insights: Beyond medical devices, Ypsomed observes regulations in related sectors, such as pharmaceuticals and chemicals, to identify relevant best practices.
- Early Requirement Analysis: Ypsomed’s teams provide timely insights into new and emerging regulations, ensuring that potential impacts are considered early in product development.
- Continuous Adaptation of Processes: Ypsomed’s internal procedures are regularly updated to reflect the latest guidelines and regulatory requirements.
A key strength of Ypsomed’s strategy is the active participation of subject matter experts in international standardisation committees and industry associations. This involvement enables the company not only to contribute its expertise, such as in the enhancement of the ISO 11608 series of regulations for needle-based injection systems, but also to anticipate emerging priorities, such as user-centric design and sustainability, well before they become formal requirements.
For example, when health authorities began placing greater emphasis on human factors data, Ypsomed was able to respond proactively. Its regulatory affairs and usability engineering teams co-developed study protocols that directly addressed both US FDA and EMA expectations. This ability to adapt drug delivery platforms in real time demonstrates how regulatory intelligence can support compliance while fostering innovation and market readiness.
INNOVATION BEHIND TECHNOLOGY: PLATFORMS THAT EVOLVE
At Ypsomed, innovation is not just about developing new devices or technologies. It is defined by how drug delivery solutions integrate with evolving therapies, regulatory frameworks and the reality of real-world applications. The shift from hospitals to the home illustrates this perfectly. Patients now expect devices that are simple, easy to use, reliable and increasingly connected – a demand that is reshaping both technical and regulatory pathways.
Ypsomed’s innovation strategy is closely tied to its configurable, standardised platform technologies for injection pens, autoinjectors and patch injectors. Each new project builds on a foundation of proven expertise, transferring learnings and regulatory insights from previous projects. This platform approach ensures that when therapeutic requirement changes, such as high viscosities or large volumes, devices can be adapted without unnecessary compliance or development risk. Ypsomed engineers platform modularity from the outset with flexibility in mind, supported by regulatory insight, making subsequent adaptation and scaling far more straightforward.
THE POWER OF PLATFORM MODULARITY
“A COMBINATION OF PROVEN PLATFORMS AND ROBUST TECHNICAL DOCUMENTATION ENABLES REGULATORY AFFAIRS, TOGETHER WITH THE PROJECT TEAM AND QUALITY COLLEAGUES,
TO SUPPORT OR MANAGE MULTIPLE SUBMISSIONS FOR DRUG-DEVICE COMBINATION PRODUCTS AND MEDICAL DEVICES IN PARALLEL.”
As platform components are already characterised and validated in manufacturing, new projects can focus on therapy-specific adaptations rather than re-proving fundamentals, accelerating the development process. A combination of proven platforms and robust technical documentation enables regulatory affairs, together with the project team and quality colleagues, to support or manage multiple submissions for drug-device combination products and medical devices in parallel. Ypsomed’s platform documentation is maintained as “living regulatory files”, being continuously updated with authority feedback and systematic voice-of-customer input.
Overall, Ypsomed’s innovation process combines collaboration, multidisciplinary expertise and transparency. With regulatory professionals embedded from the earliest concept stage, new developments are de-risked, regulatory strategies are strengthened and submissions become more predictable and timely worldwide.
FROM INNOVATION AND DEVELOPMENT TO GLOBAL REGULATORY READINESS
Ypsomed manages the transition from innovation to product development with precision, guided by its certified quality management system and global regulatory frameworks. Aside from regulatory affairs specialists embedded across the organisation, several functions contribute to a compliant and efficient pathway from concept to market:
- R&D and Engineering: Align device concepts with regulatory pathways from the earliest stages.
- Human Factors & Usability: Design and conduct formative and summative studies that meet the expectations of regulatory authorities.
- Risk Management: Define and integrate risk mitigation measures into documentation and processes from the very beginning.
- Manufacturing and Supply Chain: Anticipate requirements for scale-up, localisation and traceability.
- Marketing and Communications: Align labelling, claims and global launch strategies.
- Business Development: Translate regulatory insights into value propositions, partner strategies and market-entry opportunities.
Throughout the development process, comprehensive documentation is prepared to ensure that all safety and performance requirements are consistently fulfilled. These records provide clear traceability to supporting test data and risk analyses, while also taking regional regulatory expectations into account.
Anticipating Authority Expectations
With decades of experience across drug-device combination products and medical devices, Ypsomed applies its deep regulatory expertise to anticipate authority expectations, identify trends and resolve contradictions before they impact partner projects. In this way, Ypsomed ensures that development is not only innovative and efficient, but also submission-ready and highly standardised, enabling timely approvals and reliable market access.
“YPSOMED REDUCES THE REGULATORY WORKLOAD FOR ITS PARTNERS AND HELPS TO ENSURE SMOOTH, COMPLIANT MARKET CONTINUITY.”
Lifecycle Confidence Through Regulatory Continuity
Regulatory responsibilities do not end with approval; they extend throughout the entire product lifecycle (Figure 4). Ypsomed’s lifecycle management approach is driven by robust and agile change-control processes, rigorous updates and surveillance programmes, and a commitment to continuous improvement. By taking responsibility for ongoing file maintenance, implementing technical updates in line with evolving requirements and managing interactions with health authorities, Ypsomed reduces the regulatory workload for its partners and helps to ensure smooth, compliant market continuity.
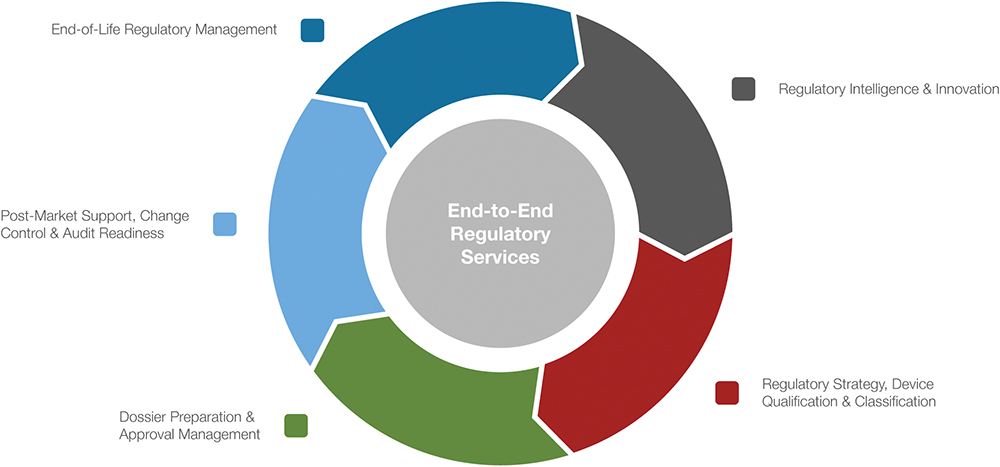
Figure 4: From innovation to end-of-life – regulatory leadership at every step.
One of Ypsomed’s strengths is its ability to co-ordinate multi-regional launches, either in parallel or in sequence, in the US, EU, China, India, Japan and other markets (Figure 5). Local regulatory teams, complemented by manufacturing and supply chain hubs, such as the company’s strategic presence in China, ensure region-specific requirements are met and that local market expectations are addressed.
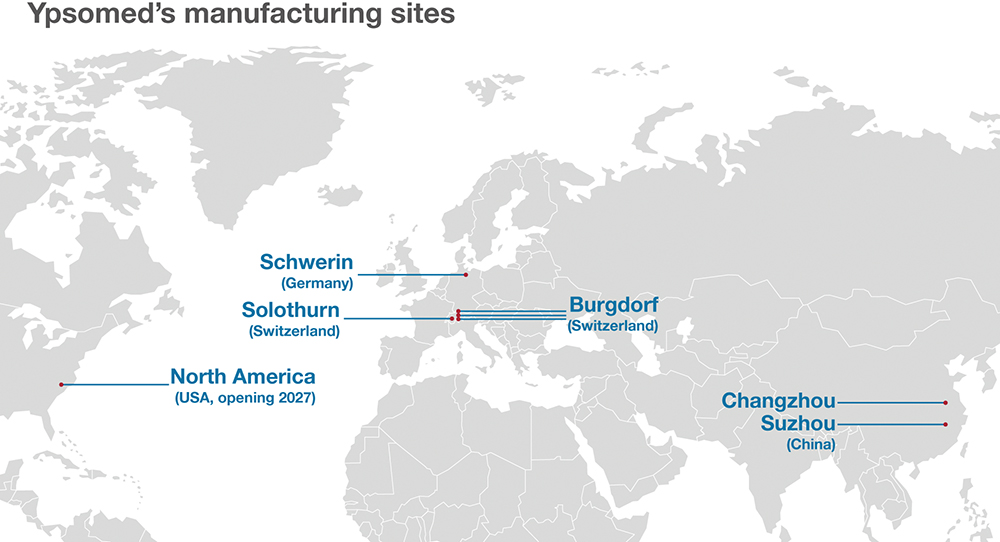
Figure 5: Ypsomed’s global footprint.
To further strengthen this global footprint, Ypsomed is also preparing to establish a physical presence in the US. This step carries strategic importance by:
- Placing Ypsomed closer to its customers
- Enabling faster response to market expectations
- Mitigating the impact of regional factors, such as pricing pressures and tax frameworks.
Together with its European and Asian sites, a US hub will complete Ypsomed’s global network – ensuring agile, compliant and cost-efficient access to all major healthcare markets.
Embedding Evolving Requirements
Ypsomed seamlessly embeds changes to regulations and requirements into its platform portfolio, from revised technical standards to growing sustainability demands. In parallel, a continuous stream of global submissions is supported by an ongoing and structured dialogue with health authorities, enabling the company to remain aligned with current expectations and to anticipate forthcoming regulatory developments.
Managing End-of-Life Responsibility
The end of a product’s lifecycle is not just discontinuation. Regulatory compliance and partner obligations must remain aligned until final market withdrawal. Ypsomed’s regulatory affairs experts ensure that documentation, labelling and authority requirements are maintained, providing predictability and transparency. In this way, Ypsomed’s regulatory involvement spans the entire journey of a product, from innovation and development through global submissions and market access to end of life, ensuring consistent regulatory excellence at every stage.
WHAT SETS YPSOMED APART
Ypsomed’s strengths in regulatory expertise stem not from a single process or capability, but from a unique combination of enduring factors:
- Deep collaboration with pharma partners
- High submission volume support, giving nuanced insight into how regulatory trends are enforced in practice
- Timely implementation of new requirements
- Organisational agility based on continuous improvement and learning.
This readiness, built on core values of innovation, platform flexibility, operational rigor and a genuine sense of responsibility, enables Ypsomed to anticipate the needs of both regulatory authorities and partners, providing a strong foundation for building long-term trust and value.
TURNING COMPLEXITY INTO OPPORTUNITY
In a world where drug delivery technology, regulatory expectations and patient needs are all in continuous motion, success is not guaranteed by simple compliance. It requires anticipation, adaptation and leadership. For Ypsomed, this means embedding regulatory excellence at every stage, from the very first brainstorm of a new idea through project development and market launch to end of life.
With its regulatory compass, Ypsomed transforms difficulty into opportunity. With decades of expertise, trusted services and a platform-based approach, the company steers through complex issues to ensure that products are market-ready, compliant and aligned with future trends on the horizon. By embedding regulatory excellence at every stage, Ypsomed supports improved patient outcomes and delivers long-term value for partners.

