To Issue 178
Citation: Barichello E, Sutton C N, “Proving the Design Space: Advancing the Development of Syringe-Device Combinations”. ONdrugDelivery, Issue 178 (Oct 2025), pp 10–15.
Enrico Barichello and Courtney Nicholas Sutton describe the innovative combination of SHL‘s Molly® autoinjector with Stevanato‘s Alba® and Nexa® prefilled syringes, which addresses the need to streamline device approval by establishing comprehensive compatibility testing, collaboration between suppliers and validated solutions for drug delivery.
The increased use of biologic medicines and other therapeutic innovations for treating chronic and serious diseases has been accompanied by growing demand for at-home administration. Drug-device combination products, such as prefilled syringes (PFSs), autoinjectors and other drug delivery systems, have evolved to meet this demand. Although these products have been used for over two decades by patients and caregivers with great success, drug developers still seek to improve the patient experience. Reducing injection frequency is one such path to improvement. A key challenge to less frequent dosing is balancing drug characteristics – such as viscosity, which depends on drug concentration and the required dosage – with the performance capability of devices. In autoinjectors, for instance, achieving this balance can affect the injection time, which significantly contributes to the usability of the final drug delivery system.
Historically, the task of successfully integrating drug and device has fallen to the pharmaceutical companies developing these drugs. However, container closure system (CCS) and delivery device manufacturers play a critical role in supporting the development of these products. As such, there is an opportunity for manufacturers to collaborate and thus de-risk the pathway from investigational drug to approved product.
One approach is to determine the design space in advance with a thorough test strategy. Drug developers can then assess system compatibility with their drugs using robust data, giving them greater confidence during their formal primary container and autoinjector selection processes. The adoption of platform technologies via such a pathway can shorten time to clinic and, ultimately, time-to-market when applied across a pharmaceutical company’s pipeline.
This article outlines market trends in patient-centric, self-administered injectable drugs and discusses how primary container and device technology developers can work together to de-risk the task of bringing such products to market.
“BIOLOGIC THERAPIES IN PARTICULAR ARE RESHAPING TREATMENT LANDSCAPES DUE TO ADVANCES IN MOLECULAR BIOLOGY AND THE RISING PREVALENCE OF DISEASES IN SEVERAL DIMENSIONS.”
MARKET TRENDS AND INNOVATION
As new therapies emerge, drug delivery methods evolve to improve patient outcomes. The current focus is on more effective, intuitive and convenient injectable drugs, including biologics and other high-value treatments that patients can self-administer at home, improving adherence and the overall treatment experience. Biologic therapies in particular are reshaping treatment landscapes due to advances in molecular biology and the rising prevalence of diseases in several dimensions, including chronic, immune-mediated, rare and oncological conditions. Other injectable drugs similarly benefit from innovations in patient-centric delivery systems.
CHALLENGES OF DEVELOPING DRUG DELIVERY SYSTEMS
Drug delivery systems that integrate drugs, CCSs and delivery devices can be challenging to develop quickly and efficiently. These challenges arise because the usual development pathway has not yet been streamlined to meet the growing need for home-administered therapies (Figure 1). Pharmaceutical developers increasingly look beyond traditional vials and manual PFS injections in favour of autoinjector presentations, which are recognised as easier and safer for at-home use.
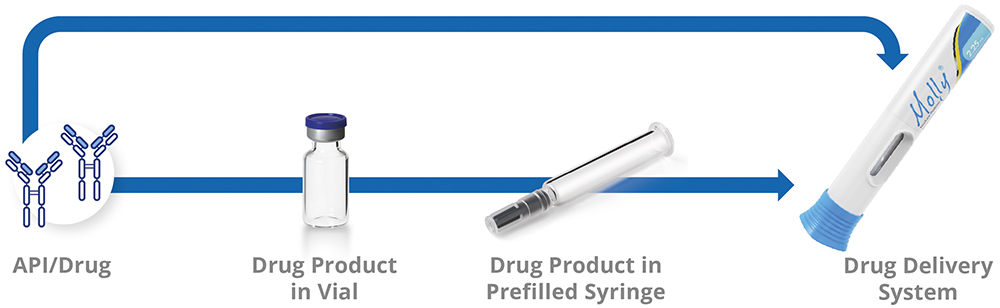
Figure 1: Main steps in combination product development.
When developing systems such as PFSs and autoinjectors, it is essential to identify the critical parameters that could affect both product quality and patient safety. Some of the key factors manufacturers must address include:
Dimensional and Mechanical Compatibility
- Ensuring that PFSs fit reliably in the delivery device
- Reducing high reject rates during final assembly
- Preventing issues such as the rigid needle shield (RNS) not being removed or cap removal forces being too high
- Minimising variability in injection depth
- Maintaining favourable force and stress profiles to prevent device or syringe breakage.
Functionality and Usability
- Reducing the injection time of viscous formulations
- Avoiding incomplete injections, stalls or failures to start
- Reducing the risk of premature removal due to slow injection and confusion over whether the injection is complete.
Drug Stability
- Preventing protein aggregation caused by silicone oil
- Limiting interactions from multiple contact materials to control extractables and leachables
- Ensuring container closure integrity (e.g. avoiding plunger movement that could impact sterility or headspace conditions).
These challenges underscore the importance of robust performance and compatibility testing. Demonstrating that the drug, container and device function together reliably is critical for pharmaceutical companies to streamline regulatory approvals, reduce development risk and gain a competitive edge. Pharmaceutical companies require this evidence before selecting a solution for an individual drug, and it can be especially useful when choosing a platform solution to support their pipeline of products.
DE-RISKING THROUGH PLATFORM INTEGRATION
The collaboration between SHL Medical and Stevanato Group demonstrates a platform approach to de-risking. By combining the Molly® autoinjector with Alba® and Nexa® PFSs, the partners provide performance data across critical attributes – cap removal force, activation force, injection time, needle extension and dose accuracy.
This approach demonstrates robust compatibility across multiple formulations, reduces stock-keeping unit complexity and simplifies container-device integration. By using a platform-based solution with multiple options with regards to product performance, pharmaceutical partners can accommodate variability in pipeline products, reduce development and registration complexity, and accelerate time-to-market for high-value, home-administered injectable drugs.
BUILDING FROM A STRONG PLATFORM
For an autoinjector to function safely and effectively in patients’ hands, multiple critical design factors must be considered during development. Among these, compatibility between the drug container and delivery device is paramount.
“SHL MEDICAL AND STEVANATO GROUP BRING A LONG TRACK RECORD OF JOINTLY DEVELOPING COMMERCIALLY AVAILABLE DRUG DELIVERY SYSTEMS, WITH NINE PRODUCTS SUCCESSFULLY COMMERCIALISED IN A STEVANATO GROUP SYRINGE WITH THE MOLLY® AUTOINJECTOR
TO DATE.”
SHL Medical and Stevanato Group bring a long track record of jointly developing commercially available drug delivery systems, with nine products successfully commercialised in a Stevanato Group syringe with the Molly® autoinjector to date. This experience allows them to apply pre-existing knowledge of container-device compatibility to new projects. This foundation provides a proven platform that mitigates risk and supports consistent performance across a range of formulations.

Figure 2: PFS critical interface dimensions for autoinjector compatibility.
DIMENSIONAL FIT
Alba® and Nexa®PFSs can be reliably integrated into autoinjector platforms, including SHL Medical devices (Figure 2). Key design features include:
- Tightly controlled dimensional tolerances and optimised barrel geometries for consistent fit and secure assembly
- Carefully defined critical dimensional parameters – overall syringe length without closure (L1), length above the shoulder (L2) and total length including the RNS (L3) – to support accurate injection depth and proper engagement during device assembly
- Controlled axial alignment between the RNS and syringe barrel, ensuring repeatable performance across automated assembly processes.
These design elements collectively minimise mechanical risk, enhance assembly reliability and ensure that the syringe-device interface performs consistently – even under the stress of high-viscosity formulations.
FUNCTIONALITY
High-viscosity biologics present a significant challenge for self-injection devices. As viscosity increases, so does the force required to push the fluid through the needle, which can result in long injection times, incomplete injections, or delivery failures. Poorly designed devices may experience stalls, delayed injection start or premature removal, all of which can negatively impact patient experience, adherence and treatment outcomes.
The key challenge, therefore, is to demonstrate that the combination system can reliably deliver highly viscous biologics while maintaining acceptable injection forces and timing, ensuring accurate dose delivery and enabling easier self-administration.
To address this, Stevanato Group and SHL Medical conducted a rigorous compatibility study, illustrated in Table 1. The evaluation involved laboratory testing under simulated real-world conditions, including standard storage, clinical use and handling mishaps.
| Device | PFS | Needle | RNS | Plunger | Viscosities |
| 2.25 mL Molly® | Alba® (Cross-Linked) |
8 mm 27G sTW | RNS #1 | PremiumCoat Plunger by Aptar Pharma | 1, 15, 30, 50 cP |
| Nexa® (Sprayed-On Silicone Oil) |
8 mm 27G sTW | RNS #1 | |||
| ½” 27G sTW | RNS #2 | ||||
| ½” 27G sTW | RNS #2 | ||||
| ½” 27G sTW | RNS #2 |
Table 1: Study design configuration table.
Study Design
Syringes were filled with placebo formulations spanning viscosities from 1 cP (water-like) to 50 cP (representing highly viscous biologics). These syringes were then incorporated into SHL Medical’s Molly® autoinjector devices. Performance tests were conducted at room temperature (RT), after cold storage (2–8°C), and following a one-metre free-fall drop in compliance with ISO 11608-1.
Outcomes
The integrated system successfully administered formulations up to 50 cP viscosity with injection times increasing linearly and predictably in correlation with viscosity. As shown in Figure 3, the injection times in the optimised category Alba® and Nexa® 8 mm 27G sTW (special thin-wall), as well as Nexa 25G sTW ½” (12.7 mm), were recorded at under 20 seconds with high-viscosity solutions up to 50 cP. These results highlight the system’s ability to maintain efficient delivery across a range of formulation viscosities while using sTW needle technology.
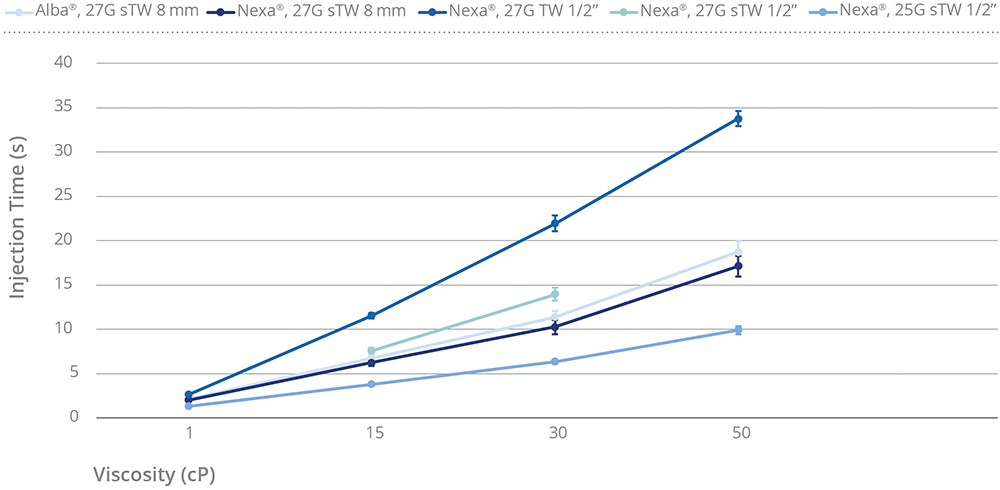
Figure 3: Injection time versus viscosity.
Alba® 27G sTW 8 mm demonstrated slightly higher injection time compared with Nexa® 27G sTW 8 mm, which can be attributed to the structure of its cross-linked silicone layer.
Figure 4 illustrates how injection times are reduced when using optimised needle gauges and lengths compared with the standard ½” 27G thin-wall configuration. The 8 mm 27G sTW configuration consistently delivered shorter injection times relative to the ½” 27G TW reference.
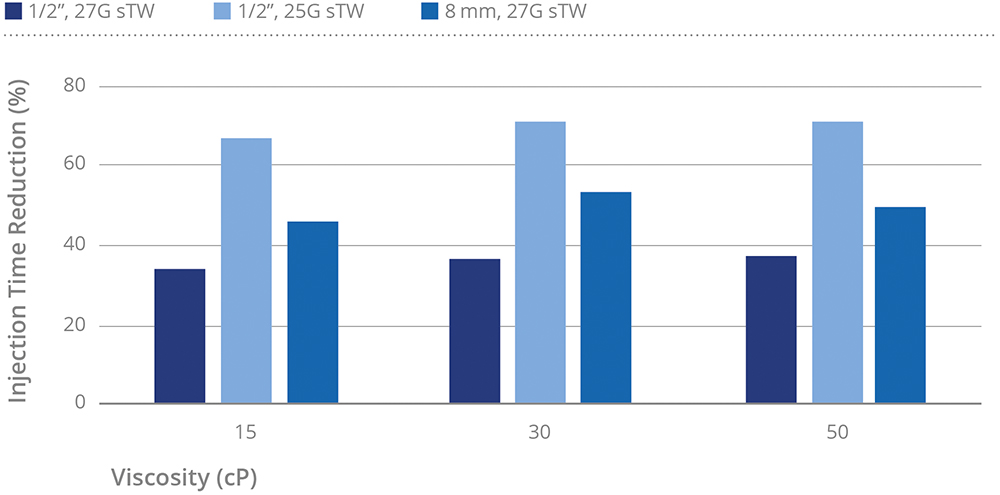
Figure 4: Injection time reduction relative to 27G TW ½”. Note: For the 27G sTW categories at 50 cP, the values were derived from empirical measurements obtained in previous experimental studies.
At low viscosity (1 cP), this reduction in injection time was modest, around 25% (data not shown). For higher viscosity formulations (15–50 cP), the 8 mm 27G sTW needle achieved greater reductions, approximately 45–55%. The 25G sTW, with its larger inner diameter, allowed for more pronounced optimisation, with a reduction of 65–70%. The 27G sTW also demonstrated improvement, with injection time reductions of approximately 35%, attributable to the optimised inner diameter while maintaining the same external diameter. Collectively, these findings highlight the advantages of shorter, optimised needle designs in enhancing injection efficiency, particularly for viscous biologic formulations.
When uncapping an autoinjector, high removal forces may make it difficult for the user to remove the cap. If the removal force is too low, the cap may fall off prematurely during shipping or handling and cause problems for the end user.
The study measured autoinjector cap removal for two commercial RNSs with needle gauges of 27G and 25G (Figure 5):
- All values were below the industry-accepted upper specification limit (35 N)
- RNS #2 displayed lower values reflecting the different composition of the elastomer
- Variability was in a comparable range, irrespective of the RNS and needle gauge.
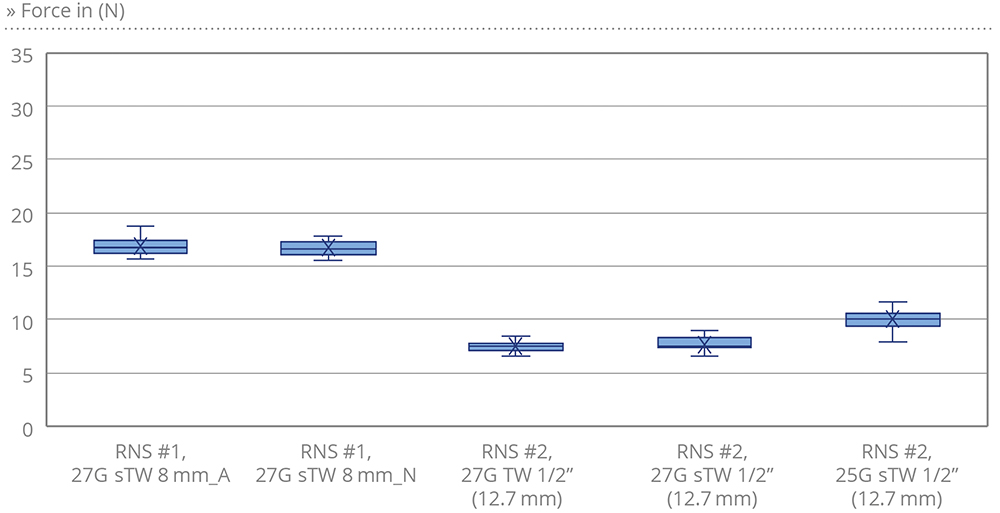
Figure 5: Cap removal force (N).
All tested performance attributes were within predefined specifications, confirming the robustness of the Molly® platform and Stevanato Group syringe.
Activation forces were syringe-independent and demonstrated stability across the tested temperature conditions, confirming the robustness of device firing performance under varying environmental scenarios. Needle extension was reliable and met specifications across different needle types.
DRUG STABILITY
Maintaining drug stability within the primary container is a critical consideration in the development of drug delivery systems for biologics and other sensitive injectables. Conventional PFSs typically rely on silicone oil as a lubricant. However, silicone oil can interact with protein-based formulations, promoting aggregation or denaturation. These instabilities may adversely affect drug safety, efficacy and shelf life.
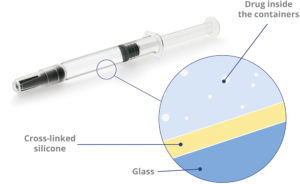
Figure 6: Alba® glass syringes: advanced coating for enhanced stability of sensitive drugs.
To mitigate these risks, Stevanato Group has developed the Alba® syringe platform, which incorporates an internal cross-linked silicone coating (Figure 6). This design substantially reduces the amount of free silicone oil in contact with the drug product, thereby lowering the risk of protein aggregation and particle formation. At the same time, the coating maintains the functional performance required for injection, including stable glide forces and consistent break-loose behaviour.
The Alba® and Nexa® Syringe Platforms
The combination of Alba® and Nexa® syringes with SHL Medical’s autoinjector technology provides a robust solution for sensitive biologics. The reduction in silicone-drug interactions contributes to consistent injection times and reliable dose delivery, supporting device performance across a wide range of formulations.
THE PATIENT PERSPECTIVE
For patients managing chronic conditions such as rheumatoid arthritis, multiple sclerosis or diabetes, ease of use and comfort are paramount if we are to expect them to remain adherent. Reduced dexterity complicates device operation, underscoring the importance of predictable actuation forces and manageable preparation steps.
“STEVANATO GROUP AND SHL MEDICAL PROVIDE A COMPATIBLE CONTAINER-DEVICE PLATFORM THAT MAINTAINS CAP AND NEEDLE-SHIELD REMOVAL FORCES WITHIN A RANGE THAT ENSURES CLOSURE INTEGRITY BUT REMAINS OPERABLE WITHOUT EXCESSIVE PATIENT EFFORT.”
Stevanato Group and SHL Medical provide a compatible container-device platform that maintains cap and needle-shield removal forces within a range that ensures closure integrity but remains operable without excessive patient effort. Activation force consistency assures dependable autoinjector function, reducing frustration and the risk of partial dosing. Injection times remain in a range that can be injected tolerably and simultaneously enable comfortable holding of the device against the skin to ensure a complete injection.
CONCLUSION
Compatibility tests on the Nexa® and Alba®2.25 mL PFSs with the Molly® 2.25 mL autoinjector confirm full compatibility between these components. In combination, they reliably deliver high-viscosity biologics with predictable injection times, consistent mechanical performance and accurate dosing.
By using pre-verified solutions such as this, drug developers can simplify container and device selection, reduce development timelines and lower integration risks – accelerating time-to-market (Figure 7). The combined expertise of Stevanato Group and SHL Medical supports complex drug delivery projects, offering a practical, de-risked approach for pharmaceutical partners. Together, these platform-based solutions illustrate how co-development and integrated expertise can streamline the path to patient-centric biologic therapies.
Figure 7: Risk mitigation strategies.
SHL, the SHL Medical logo and Molly® are registered and/or non-registered trademarks of SHL Medical AG or its affiliates.
Figure 2, featuring 2.25 mL syringes with PremiumCoat® plungers, provided courtesy of Aptar Pharma.

