To Issue 131
Citation: Solanki A, Bhat J, Kalafat J, “Capsules for Dry Powder Inhalation – a Complete Product”. ONdrugDelivery, Issue 131 (Apr 2022), pp 76–80.
Anita Solanki, Jnanadeva Bhat and Justin Kalafat discuss capsules for dry powder formulation and consider their potential as a dosage form for patients with respiratory conditions and systemic illnesses.
“The inhalation route has numerous benefits over the conventional oral route, such as lower doses, matchable therapeutic efficacy
and reduced side effects.”
There has been a substantial rise in the prevalence of chronic respiratory diseases globally1 and chronic obstructive pulmonary disease (COPD) is now the third leading cause of death worldwide.2 This growth trend, combined with advances in applications of systemic pulmonary drug delivery for non-respiratory conditions, means that inhalation drug delivery is an increasingly important area for research and development, and there is a clear need to develop better, more effective and affordable inhalation methods.3
The inhalation route has numerous benefits over the conventional oral route, such as lower doses, matchable therapeutic efficacy and reduced side effects.4 Extremely sensitive molecules, potent molecules and larger molecules, such as proteins and peptides, can be delivered via inhalation.5 For systemic delivery, the higher vascular nature and larger surface area of the lungs mean that drug absorption into the patient’s systemic circulation is effective and without first-pass metabolism.
The efficiency of drug delivery to the patient by inhalation ranges between 12 and 40%6 of the dose inhaled. The remainder is expelled or deposited in the upper respiratory tract. However, in the treatment of respiratory diseases and other conditions affecting the lungs, the drug is being targeted to the site of action, meaning that a lower dose can be administered than would be required if it were taken orally, resulting in fewer side effects. For example, the oral dose of salbutamol (albuterol) is 2–4 mg, whereas the inhaled dose ranges from 0.2–0.4 mg, which is one-tenth of the oral dose. Additionally, the onset of effect is often faster when drugs are inhaled compared with oral administration. With an oral solid dose of salbutamol, the onset of effect is about 30 minutes, whereas with inhalation it is about five minutes. These clear benefits contribute to the growth in popularity of the inhalation route.
Nebulisers, pressurised metered dose inhalers (pMDIs) and dry powder inhalers (DPIs) are the three primary types of inhalation drug delivery system.7 As well as the devices, the formulations used are different for each type.
Nebulisers are used mostly in hospital settings and often require the supervision of healthcare personnel. Due to this and their size, nebulisers are not frequently used for the routine treatment of chronic respiratory diseases.
In pMDIs, the drug is dissolved or suspended in a propellant. When the device is activated, the drug aerosolises and is deposited in the lungs. The propellants in pMDIs have caused concern regarding environmental damage. Early devices used chlorofluorocarbons (CFCs), which damage the ozone layer. In response, pMDIs with hydrofluorocarbon (HFC) propellants that do not damage the ozone layer were developed. However, the HFCs being used were found to be greenhouse gases.8 Less harmful HFC propellants are being developed and beginning to reach the market.
Other potential challenges arising with pMDIs include non-uniformity of emitted dose if patients are not given correct use guidelines,9 and the requirement for the patient to co-ordinate actuation of the device using their hand with their inhalation of the drug.
“Hard gelatin capsules and HPMC capsules act as pre-metered unitdose systems, and both offer easy encapsulation for formulations.”
DRY POWDER INHALATION
In ACG’s opinion, dry powder inhalation technology is the most promising approach for numerous future applications both in the treatment of a range of respiratory conditions and other diseases affecting the lungs, and for systemic drug delivery via the lung. DPIs are already an incredibly important method of drug administration. Since the restriction of CFCs in the late 1980s, DPIs have become the most frequently used type of inhaler, and the preferred inhalation device technology in many European nations.10
Both single- and multidose DPIs are available in the market. The global DPI market is projected to rise to US$1.3 billion (£991 million) by 2031, growing at a compound annual growth rate of 7.2% (2021–2031). The market is predicted to generate opportunities of approximately $458 million in the next 10 years. DPIs account for more than 35% of the global inhaler market.11 Capsule-based inhalers are likely to lead the market due to a rise in the number of companies offering inhalation products and the increase in acceptance of these devices by healthcare personnel and patients.
Capsule-based DPIs (cDPIs) are the most popular method of administration as they are propellant-free, provide uniform dose delivery and offer patient-centric portability and ease of operation.12 The effective performance of products is dependent on the capsule, inhalation device, formulation and attributes such as particle size, distribution, shape and surface properties. As breath-device co-ordination is not required, cDPIs are suitable for patients of all ages and respiratory abilities.
CAPSULES FOR DRY POWDER INHALERS

Figure 1: Capsule for dry powder formulation.
cDPIs are portable, easy to use and control, cost-effective, painless and, most importantly, patient-friendly. Capsules for DPIs are specifically designed for this application and are different from regular oral capsules. Different process parameters, including environmental conditions and additives, are employed during the manufacture of inhalation capsules because the capsule, as the primary container of the formulation, contributes to powder aerosolisation of the micronised drug from the carrier during inhalation. Critical attributes of this type of capsule are low residual lubricant (for lower powder retention), microbial limits and capsule-device compatibility (Figure 1).
Residual lubricant in the capsule is one of the key factors that must be managed to ensure low powder retention in the capsules after the formulation is inhaled via the device. Therefore, the edible lubricant used on the capsule pin bars during manufacture is optimised and the quantity is controlled. The quantity of powder retained inside the capsule is dependent on the interaction between the inner surface properties of the capsule and the characteristics of the powder formulation. Conventional oral capsules do not have optimised or controlled lubricant content, hence the powder retention is higher than in capsules for DPIs (Figures 2 and 3).
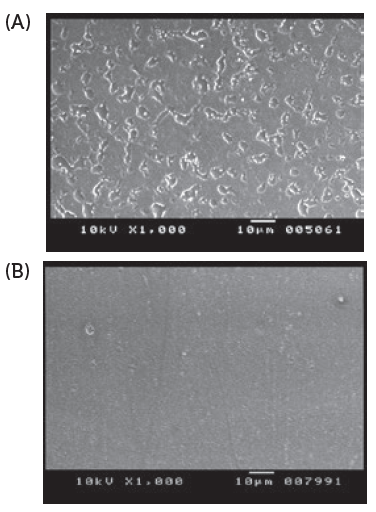
Figure 2: Scanning electron microscopy imaging of internal surface of capsules. Conventional oral capsules show larger lubricant globules (A), and cDPI uniform layer of lubricant (no large globules) (B).
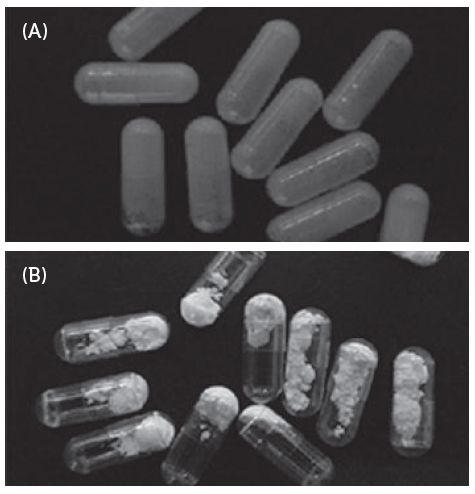
Figure 3: Powder stuck to inner surface of oral capsules(A) and Free flowing powder in cDPI (B).
With inhalation, the microbial limit is an essential criterion in capsule manufacturing. The respiratory tract is susceptible to contamination and subsequent infection, unlike oral delivery where harsh gastric acid helps prevent certain infections. This is why the acceptable microbiological limits for DPI capsules are more stringent than for oral capsules and should be no more than 100 CFU/g.13

Figure 4: Partially
filled cDPI.
HPMC CAPSULE SUITABILITY FOR INHALATION APPLICATION
Two-piece hard capsules are manufactured from either gelatin or hydroxypropyl methylcellulose (HPMC) – also known as hypromellose. Both the polymers have different characteristics that can impact the performance of the finished product. Globally, the most widely used capsule size for commercial products is size 3. Sizes 2 and 0 are also being taken into consideration in the development cycle for new products. cDPI capsules are partially filled (Figure 4).
HPMC capsules have an inherently lower moisture content of about 3%–8% w/w while gelatin capsules have around 13%–16% w/w. The puncturing properties of HPMC capsules are better over a wider range of relative humidity than gelatin capsules because HPMC capsules are more resistant to deformation.14 HPMC polymer capsules are stable, inert and flexible; thus they do not face brittleness challenges when being punctured. HPMC capsules have exhibited outstanding properties in the stability of formulations due to lower moisture content and aerosolisation.15
“The future development of cDPI formulations and devices must focus on newer therapeutic segments and newer molecules to be delivered via inhalation. ”
DRY POWDER INHALATION FORMULATION
Commonly, dry powder inhalation formulations are a mixture of the micronised drug and a carrier, for example, inhalation-grade lactose. Carrier particles are used to improve flow, dosing accuracy and overall fill weight for low doses. This facilitates manufacturing processes by improving the handling and encapsulation of the powder formulation.
For the drug component, potential critical attributes include particle size distribution, moisture content, bulk density, flow properties, morphic form (e.g. amorphous, crystalline, hydrate), the morphology of drug particles (e.g. shape, texture, surface area) and impurities.
Pertinent properties for consideration while selecting carriers during product development include the ratio of drug to carrier, compatibility and particle size distribution. Inter-particle interactions between the drug, carrier and device constituent, as well as cohesive and adhesive forces, surface area and static charge properties of the formulation, are also important. If not properly managed, these properties may affect uniformity, flow property and dose delivery.
The drug particles are adsorbed on the carrier surface and then detached by the inhaled air stream. Inhaled air energy is greater than the adhesion forces between the drug and the carrier particle causing deagglomeration.16 The turbulent airflow then leads to the deposition of the drug in the lungs. The aerodynamic diameter of drug particles controls the drug deposition. Generally, particles with aerodynamic diameters between 1 and 5 μm are more likely to deposit in the lungs effectively.17
INHALATION DEVICE COMPATIBILITY
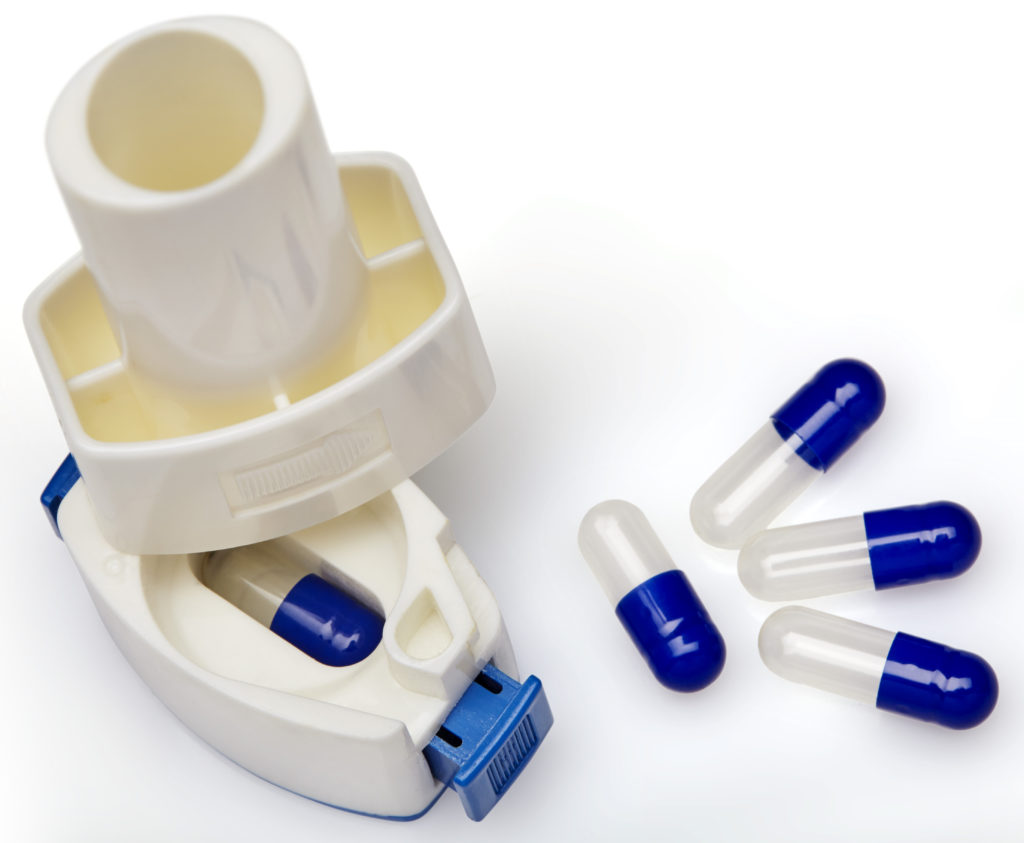
Figure 5: Capsule puncturing device.
Device design is fundamental to effective drug delivery for cDPIs. It is critical that the patient can produce adequate air flow to inhale the drug from the capsule, to deliver it to the lungs and achieve the desired therapeutic effect. An ideal inhaler would provide uniform dosage and be easy for patients to use and carry. As devices may come into direct contact with the formulation, they have the potential to affect product safety and performance. For instance, drug particle-surface interactions, such as the adhesion of a drug to the surface of the mouthpiece, can affect the emitted and delivered dose. Therefore, the properties of the device material are important. The design and dimensions of the device’s components can also influence the resistance, air flow, shear and turbulence generated within the device and, therefore, impact drug delivery. Hard gelatin capsules and HPMC capsules act as pre-metered unitdose systems, and both offer easy encapsulation for formulations. Capsule activation can be achieved by twisting open the cap and body to release the formulation into the chamber of the device or by puncturing the capsule on the cap and body with pins. The puncturing technique is the more frequently used device system to release powder from the capsule. Puncturing can either be from the top (dome of cap and body) or the side of the capsule (Figure 5).
Although the puncturing behaviour under normal storage conditions has been proven acceptable for both HPMC and gelatin, HPMC capsules exhibit better performance under dry conditions. No fractures or particles from the shell are observed, and they exhibit better flexibility compared with gelatin capsules (Figure 6).
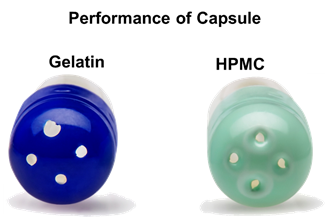
Figure 6: Gelatin capsules with uneven punctures. HPMC capsules with even punctures and no fragmentation.
NOVEL THERAPEUTIC SEGMENTS IN INHALATION ROUTE VIA CDPI
After the US FDA and the EU EMA approved the first inhaled therapeutic macromolecule for systemic delivery, human insulin, scientific researchers began exploring other opportunities for patients to benefit from inhalation delivery.18 In contrast to injection, inhalation is not associated with pain, giving the potential for improved patient compliance and, in turn, improved treatment outcomes too.19
Now, other lung diseases, such as cystic fibrosis, lung cancer, and respiratory infectious diseases, as well as systemic disorders, including diabetes, Parkinson’s disease, migraine and cancer, are being considered for treatment by dry powder inhalation drug delivery. It is not only new therapeutic segments that are being targeted but also old molecules, such as ciprofloxacin, vancomycin and sumatriptan, that could be administered via inhalation for new applications.
CONCLUSION
Already, cDPIs are demonstrating great success as a dosage form to treat patients with respiratory conditions, as well as systemic illnesses. Their potential is likely to continue to increase with new drug developments, particularly as capsules for inhalation administration are an effective and affordable option for dry powder formulations.
Soon, this dosage form will become more prevalent and will help in the treatment of a wide range of diseases. The future development of cDPI formulations and devices must focus on newer therapeutic segments and newer molecules to be delivered via inhalation.
REFERENCES
- Labaki WW, Han MK, “Chronic respiratory diseases: a global view”. The Lancet, 2020, Vol 8(6), pp 531–533.
- “Chronic obstructive pulmonary disease (COPD)”. World Health Organization, Jun 21, 2021.
- Marianecci C et al, “Pulmonary Delivery: Innovative Approaches and Perspectives”. J Biomater Nanobiotechnol, 2011, Vol 2, pp 567–575.
- Timsina M et al, “Drug delivery to the Respiratory Tract using Dry Powder Inhalers”. Int J Pharm, Vol 101 (1–2), pp 1–13.
- Hamishehkara H et al, “Pharmacokinetics and Pharmacodynamics of Controlled Release Insulin Loaded PLGA Microcapsules using Dry Powder Inhaler in Diabetic Rats”. Biopharm Drug Dispos, 2010, Vol 31, pp 189–201.
- Lavorini F, Fontana GA, Usmani OS, “New Inhaler Devices – The Good, the Bad and the Ugly”. Respiration, 2014, Vol 88(1), pp 3-15.
- Ibrahim M, Verma R, Garcia-Contreras L, “Inhalation drug delivery devices: technology update”. Med Devices (Auckl), 2015, Vol 12(8), pp 131–139.
- Myrdal P, Sheth P, Stein SW, “Advances in Metered Dose Inhaler Technology: Formulation Development”. AAPS PharmSciTech, 2014, Vol 15(2), pp 434–455.
- Chierici V et al, “Consequences of Not-Shaking and Shake-Fire Delays on the Emitted Dose of Some Commercial Solution and Suspension Pressurized Metered Dose Inhalers”. Expert Opin Drug Del, 2020, Vol 17(7), pp 1025–1039.
- Marriott C, Frijlink HW, “Lactose as a Carrier for Inhalation Products: Breathing New Life into an Old Carrier”. Adv Drug Deliv Rev, 2012, Vol 64(3), pp 217–219.
- “Dry Power Inhaler Market”. Research Report, Fact.MR, Dec 2021.
- Atkins P, “Dry Powder Inhalers: An Overview”. Respir Care, 2005, Vol 50(10), pp 1304–1312.
- Buttini F et al, “Understanding the Importance of Capsules in Dry Powder Inhalers”. Pharmaceutics, 2021, Vol 13(11), p 1936.
- Justin K, Jnanadeva B, Fernando D, “Inhalation Delivery: Using Design of Experiments (DOE) to Optimize Inhalation Capsule’s Puncturing”. AAPS, 2017.
- Al-Tabakha MM, “HPMC Capsules: Current Status and Future Prospects”. J Pharm Pharm Sci, 2010, Vol 13(3), pp 428–442.
- Hamishehkara H et al, “Effect of Carrier Morphology and Surface Characteristics on the Development of Respirable PLGA Microcapsules for Sustained-Release Pulmonary Delivery of Insulin”. Int J Pharm, 2010, Vol 389(1–2), pp 74–85.
- Sakagami M, “In vivo, in vitro and ex vivo Models to Assess Pulmonary Absorption and Disposition of Inhaled Therapeutics for Systemic Delivery”. Adv Drug Deliv Rev, 2006, Vol 58(9-10), pp 1030–1060.
- Hamishehkar H et al, “The Role of Carrier in Dry Powder Inhaler”. In “Recent Advances in Novel Drug Carrier Systems”, (Sezer AD, ed) 2012, pp 39–66.
- Laube BL, “The Expanding Role of Aerosols in Systemic Drug Delivery, Gene Therapy, and Vaccination”. Respir Care, 2005, Vol 50(9), pp 1161–1176.

