To Issue 127
Citation: Audibert R, Tunkel M, Basso M ,“Leveraging Human Factors to Develop Patient-Centric Inhalers”. ONdrugDelivery, Issue 127 (Nov 2021), pp 42-46.
Raphaële Audibert, Mark Tunkel and Manuela Basso look at the role patient-centricity has to play in the development of inhalers to treat chronic respiratory diseases.
“Traditionally, the inhalation route is used for drug administration to the respiratory system – inhalers being an effective way to administer the drug into the lungs through breathing.”
THE CHALLENGE OF DEVELOPING PATIENT-CENTRIC INHALATION DEVICES
Living with a chronic respiratory disease, such as asthma and chronic obstructive pulmonary disease (COPD), is more than a challenge, not only because these pathologies affect the airways, causing breathing difficulties, but also because correct use of the device to administer the treatment is not always easy. Almost 800 million people worldwide suffered from a chronic respiratory disease in 2018, primarily asthma and COPD.1
Asthma makes breathing difficult by causing the air passages to become narrow or blocked, especially during exacerbations. This, in turn, leads to wheezing, coughing, shortness of breath and chest tightness. At the other end of the scale, COPD is characterised by long-term breathing problems and poor airflow. Although neither disease is curable, they can be treated to help dilate airways and reduce inflammation or provide relief from coughing.
Global recommendations for the management of asthma2 and COPD3 highlight the importance of ensuring patients are adherent to their prescribed long-term dosing regimen. However, adherence remains a big challenge, in large part due to improper use of inhalation devices and a lack of patient comprehension and training.
Traditionally, the inhalation route is used for drug administration to the respiratory system – inhalers being an effective way to administer the drug into the lungs through breathing. This allows medicines to be delivered directly to the site of action, ensuring rapid absorption and rapid action, as well as a reduction in the side effects associated with oral medications. There is a comprehensive choice of inhalers in the market, the two main types being dry powder inhalers (DPIs) and pressurised metered dose inhalers (pMDIs).
DPIs deliver powder medications that can either be preloaded in the device or contained in capsules and loaded by the patient before use. The drug is released only when the patient takes a deep, fast breath in through the inhaler. With DPIs, the patient’s breathing capacity is critical in generating the desired therapeutic outcome as the dispersed powder needs to be broken into particles of the right size and deposited appropriately into the lungs.
pMDIs deliver pressurised drug contained in an aluminium canister that is fitted into a plastic body with a mouthpiece. In most instances, the medication dose is released into the lungs by pushing the canister into the mouthpiece. Co-ordination between inhalation and activation of the device is crucial to ensure a proper delivery of the drug to the lung.
Several studies have shown that patients commonly make errors in their inhaler technique, with both pMDIs and DPIs, despite advances in inhaler device technology. Analysis reveals that 31% of patients have a correct inhalation technique, 41% have an acceptable technique and 31% have a poor technique.4
Training patients in the correct use of their inhaler can reduce the number of technique errors, but it may not be sufficient to solve the problem. Better management of chronic respiratory therapies could be achieved by working on the ease-of-use of inhalers or by providing real-time feedback to the user on its technique to ensure more successful drug delivery.5 However, developing the best inhalation device to administer the most suitable treatment is technically challenging for various, equally important, reasons.
First of all, it is essential to ensure compatibility between drug and device to avoid chemical or physical (electrostatic) interactions. Good delivery performance can be achieved through working on fluid path optimisation, for instance. Specific functions may also be needed, such as a dose counter or automatic actuation. Also fundamental for the final result is to ensure the manufacturability of the device.
However, it is impossible to develop a drug delivery device without taking into account the high requirements set by regulatory guidance and standards, including new rules, recommendations and device specifications. All these elements form the basis for developing an effective inhaler, but what about user-friendliness and patient adherence?
“To design and develop a user-friendly and high-performing device that answers patients’ and technical needs, the only viable solution is to develop the device with the patients, from the early-stage phase to the final steps of validation.”
PATIENT-CENTRICITY: NOT ONLY A GROWING TREND, BUT A REAL NEED
Nemera understands the challenges encountered by patients living with chronic respiratory diseases and the difficulties of using existing inhalers correctly every day. To design and develop a user-friendly and high-performing device that answers patients’ and technical needs, the only viable solution is to develop the device with the patients, from the early-stage phase to the final steps of validation. Patients’ opinions and feedback are crucial to ensure the best results for usability, as they can offer ideas and inspiration based on their experience.
PATIENT JOURNEY AND CLINICIAN EXPERIENCE AS FOUNDATION FOR NEW DEVICE DEVELOPMENT
At the onset of establishing the functional requirements and user needs for a new device application, it is critical to fully understand the patient journey, as well any related clinical processes, to ensure that every decision made takes the patient’s needs into account. Such needs focus on adherence and the integration of new technologies into a variety of inhalation therapeutic areas. This foundation, acquired through an understanding of this journey, the patient’s interactions with the healthcare system and the healthcare provider experience, enables Nemera to capture the complete process patients go through when managing their disease – both from a self-administration standpoint and from a longitudinal perspective – as they progress with their condition and treatment through the healthcare system and their life stages (Figure 2).
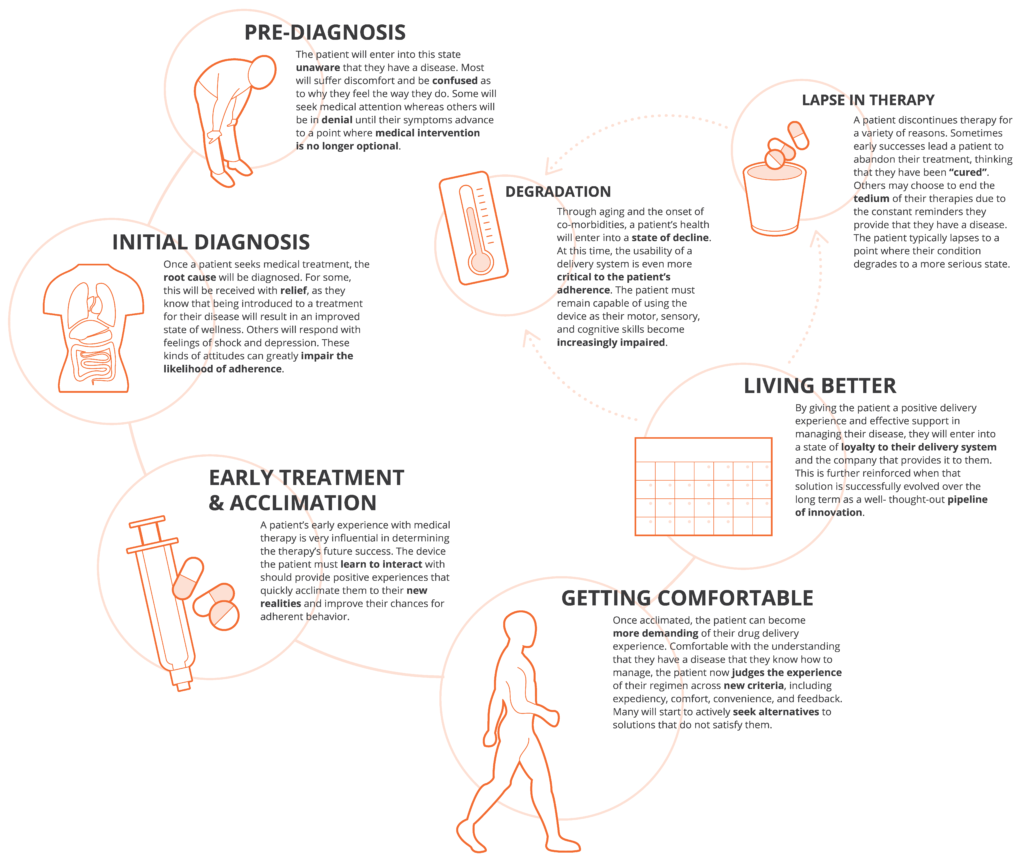
Figure 2: Key milestones along the patient journey and implications for delivery device design.
“It is very important that human factors and patient experience activities are integrated for a successful drug-device combination product development process.”
To achieve this, Nemera’s team of design research experts use a technique called applied ethnography. This method relies on a combination of interviews and in-context observations of practices, processes and experiences within the patient’s home or actual use environment. At this stage, potential use cases are looked at broadly, that is beyond the administration event or solely complying with instructions for use as you might see in a human factors study. This can potentially start from when a patient is first diagnosed, to receiving their device, and through the entire process of preparation, administration and disposal as well as the times in between treatment, so that Nemera can understand how that process changes over time and how the frequency of administration, and other factors, may impact the patient experience.
This gives the most natural view of the patient experience in relation to their environment, social/emotional context and all the other factors that influence use. It is equally important to gain an understanding of the experience of healthcare professionals (HCPs), as well as to consider this in relevant settings in clinical environments, because the diagnosis and prescription pathways for applications addressing asthma and COPD can be complex and involve many strategies for diagnosing and recommending treatment. This holistic foundation is of particular importance in inhalation applications where there are many drivers and aspects of disease-state management related to environmental factors, such as air quality and pollen levels, or co-morbidities that can impact a patient’s day-to-day use of their device. Nemera is also increasingly seeing customers consider using the inhalation modality for new therapeutic applications, such as biologics. It is therefore critical to consider the feasibility of integrating this means of delivery into a patient or clinical experience to project future state user experiences and care models.
The outputs from this work include patient journey maps, clinical process maps and a robust understanding of prioritised user needs and values, identification of pain points that can be harnessed into possibilities for improving the patient and provider experience across all aspects of the journey to make a significant impact on their lives beyond medication delivery. This can often include opportunities for integrating connectivity and electronics, both “add-on” and mobile applications, into devices to better support patients with managing their wellbeing and increasing their engagement with HCPs through information transfer and support.
This enables Nemera to consider how best to satisfy those needs as holistically as possible while making decisions around establishing user needs and functional requirements for the intended device and related drug product attributes. This includes decisions around modality, such as DPI versus MDI versus nebuliser, as well as variations within that modality, if considering existing intellectual property (IP) platforms. Nemera continually develops this foundation throughout the entire combination product journey, regardless of the device selection decisions made by its customers, through its development expertise.
It is very important that human factors and patient experience activities are integrated for a successful drug-device combination product development process. It is essential to ensure that the selected device, in combination with the drug, is appropriate, safe and effective for the target population. This also extends to optimising the patient experience to create competitive differentiation, and to ensure adherence and engagement with patients and clinical stakeholders by any means at Nemera’s disposal.
A good example of this approach might be the consideration of generic applications in a wide variety of device types (DPI, pMDI or breath-actuated MDI) where competitors are targeting the same reference drug and devices. This is compounded by the US FDA’s ANDA regulatory pathway, which requires that a generic version of a reference device must be indisguishable to a trained user from an intended use/use case standpoint as outlined in the device’s instructions for use. However, many on market devices have significant IP portfolios that must be navigated by generic players. This presents a unique challenge in which the requirements for an identical device conflict with considerations to circumvent IP and Trade Dress/Markings. A partner with broad development capabilities, such as Nemera, can apply a variety of development methods and frameworks to help customers manage these often competing requirements. Nemera can also provide consulting services for the unique human factors requirements for generic development projects, such as threshold analysis and, potentially, comparative usability studies. Balancing these requirements within the context of the development approach is critical for success.
Alternately, for NDAs and new device development programmes, the company needs to project what a future use case might look like and anticipate areas of risk to ensure that development is tailored to mitigate them. In both instances, the company needs to be sure that it is addressing the defined user groups populations and early use-related risk analysis activities to define the human factors and usability programme necessary for the intended regulatory/filing strategy. Furthermore, clinical risks must be identified through conducting formative and summative usability testing for all aspects of the device and supporting assets in alignment with the human factors programme definition, including the production of human factors engineering report documentation for use in regulatory submissions. Human factors processes must satisfy not only regulatory requirements but also lead to the development of safe, effective and differentiated combination products.
This also includes “surrounding the device” with custom support materials, such as training programmes, optimised instructions for use and other means of engagement that are critical in most inhalation applications. Nemera can use the foundation of the patient journey to anticipate key areas of clinical risk or areas in which the development of connected accessories might have value.
Nemera can offer customers consulting and development services to meet these needs. The company works closely with its customers to develop a custom human factors and user experience strategy for their combination product. In this, user adherence is taken into account to support the identified regulatory pathway with longitudinal engagement to ensure competitive differentiation. Moving forward, Nemera believes this will include developing personalised digital experiences in order to engage with patients and HCPs beyond the inhalation event, to more fully address external factors that may influence their disease-state management. When linked to the company’s capabilities in commercial manufacturing, Nemera can be a partner over the lifecycle of an application and any of its extensions.
NEMERA: FACE THE CHALLENGES AND FIND THE BEST SOLUTIONS FOR YOUR NEED
Nemera combines both technology advancement and human factors to find the most adapted solutions, allowing the improvement of both usability and performances, for improved adherence and clinical outcomes.
Best in class in inhalation drug device combination products, Nemera is recognised for its leadership position in the DPI market, and for its development and manufacturing know-how, based on strong customer references. The company has a longstanding and proven expertise in precise metering systems, design for high-speed manufacturing and dose counters co-development, as well as programme management, product development, tooling and automation. From the concept idea to large scale manufacturing, Nemera is the utmost holistic partner to develop customers’ inhalers, helping them succeed in the sprint to market.
REFERENCES
- Nemera internal analysis based on GlobalData extracts.
- “Global Strategy for Asthma Management and Prevention, (2018)”. Global Initiative for Asthma, accessed September 9, 2019.
- “Global Strategy for the Diagnosis, Management and Prevention of COPD, (2017)”. Global Initiative for Chronic Obstructive Lung Disease (GOLD), accessed September 9, 2019.
- Sanchis J, Gich I, Pedersen S and Aerosol Drug Management Improvement Team (ADMIT), “Systematic review of errors in inhaler use: has patient technique improved over time?”. Chest, 2016, Vol 150 (2), pp 394–406.
- Lavorini F et al, “Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD”. Respir Med, 2008, Vol 102(4), pp 593–604.

