Citation: Hudson-Farmer K, “Here Today and Definitely not Gone Tomorrow: Why Simplicity and Ease of Use are the Key for a New Generation of Reusable Injectors”. ONdrugDelivery, Issue 125 (Oct 2021), pp 60–66.
Kate Hudson-Farmer discusses how a new electronic reusable autoinjector – the Aria Smart Autoinjector from Phillips-Medisize – has been designed and user-tested to challenge the preconceived ease-of-use notions about electronic autoinjectors, in addition to addressing how macro trends and personal consumer subtleties could become increasingly important in creating a competitive position as devices seek to improve patient engagement.
“In previous ONdrugDelivery articles, Phillips-Medisize has argued that emerging market trends around usability and the need for connectivity are changing the market in favour of reusable electronic devices.”
THE BARE NECESSITIES AND A BIT MORE
Autoinjectors can generally be placed into one of two categories: disposable single-use and reusable. As the autoinjector market has grown rapidly over the past 15 years, it has come to be dominated by disposable single-use platform products. Such devices provide good needle safety features, as well as user feedback and cues before, during and after injection. In most situations, this design choice has appeared to offer a good trade-off between functionality, usability and cost. Additionally, whilst electronic devices have entered the market, they have tended to be more niche and bespoke, typically designed around one product for one drug by one pharma company, and not as a platform product.
In previous ONdrugDelivery articles, Phillips-Medisize has argued that emerging market trends around usability and the need for connectivity are changing the market in favour of reusable electronic devices. However, for the market to shift in this direction, it is important to show that these devices can offer safety and ease of use at least comparable to that of the leading disposable devices, as well as being cost competitive and offering improved functionality.
Reduction of use error is one of the most important goals to address when developing a new autoinjector. Accordingly, new devices require favourable results from human factors studies in order to be approved for use by the US FDA or similar bodies. Limiting the number of steps, providing clear visual and audio guidance and feedback, and limiting in-use errors and needlestick injuries are all seen as critical factors.
Use errors in particular have been extensively considered in the literature – a literature review by Weinhold et al identified 232 instances of use errors and close calls.1 Common issues cited included the inability to remove the cap, holding the device the wrong way round, removing the device from the skin too early and confusion regarding the audible and visual feedback. Newer disposable autoinjector designs have addressed many of these issues, although there is evidence that visual and audible feedback from disposable devices can be inconsistent and early lifts, resulting in wet injection, are still all too common.2 As such, although the usability of current disposable autoinjectors is good, there is still room for improvement.
“Electronic devices offer opportunities for improving injection guidance and limiting use error via a broader array of visual and audible signalling than is possible with a single-use disposable device.”
Electronic devices offer opportunities for improving injection guidance and limiting use error via a broader array of visual and audible signalling than is possible with a single-use disposable device. Although these benefits have been reported in the literature, there are also trade-offs that need to be considered, such as size, the need to load the primary drug container into the device before each injection and the presence of a user interface that may intimidate some patients even if it reassures others.
A good example of the differences in usability between current electronic and reusable devices is the work by Colier et al evaluating patient preference for the electronic autoinjector AutoTouch® compared with the disposable SureClick® device (both by Immunex, now Amgen).3 They concluded that AutoTouch® was preferred in the categories of ease of self-injection, ability to follow injection progress and providing clear confirmation of when the injection was completed, whereas SureClick® was preferred for having fewer steps and experiencing less injection site discomfort or pain. An ideal device would combine these benefits in a single design.
ARIA
Achieving such an ideal device was the objective Phillips-Medisize set itself when developing Aria, a reusable platform autoinjector. This goal required a reduction in size when compared with other electronic reusable autoinjectors, along with the drive to improve ease of use and convenience, and reduce use errors. Additionally, meeting emerging macro needs around connected health and reduced environmental impact would provide benefits, as would building in design flexibility in order to meet the needs of varying drug properties and delivery volumes.
As shown in Figure 1, Aria consists of a reusable electronic power unit, coupled with a disposable cassette that contains the prefilled syringe (PFS) and provides needle safety using a moveable shield. The cassette can accommodate both 1 mL and 2.25 mL PFSs. There are two main models, Aria, which has a simple user interface, and Aria+, which offers several advanced features, including a graphical user interface. Both models include Bluetooth connectivity.
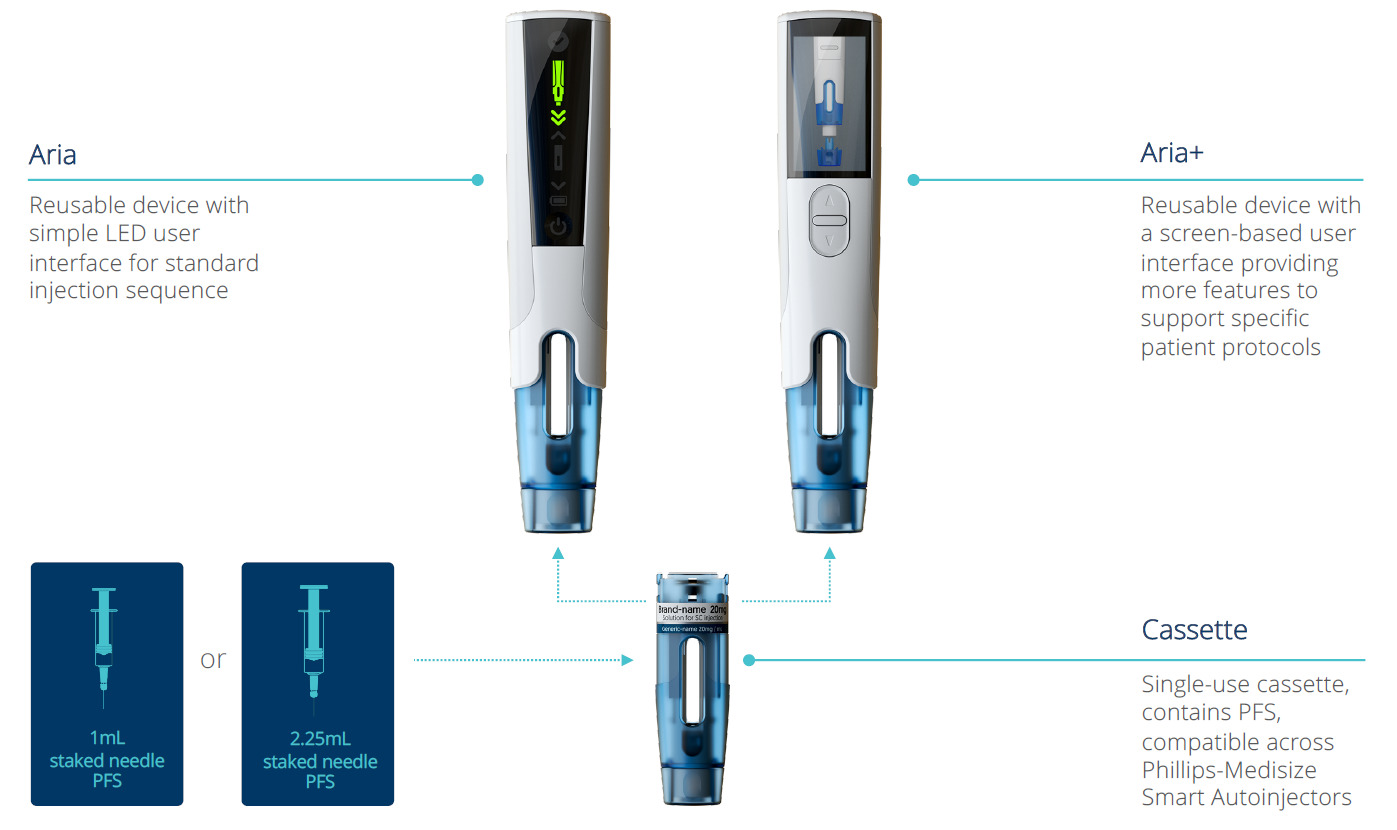
Figure 1: The Aria and Aria+ autoinjectors.
In order to gather user input during the design process, Phillips-Medisize has conducted four studies over the last two years, involving over 50 participants, including both experienced and injection-naïve users. These studies assessed how Aria addresses usability, user interface, device design, packaging and instructions for use, and how Aria compares with disposable autoinjectors in these areas (Table 1). Phillips-Medisize has also investigated the benefits of connectivity and apps, and perceptions around sustainability.
| Aspect | Aria versus disposable | Comments |
| Audible and visual signaling during injection | Enhanced | Aria has sound and visual light indication for injection progress and clear green light and check mark for dose completion with an associated audible sound – dwell time is included in the signalling so no manual counting is required for dwell time |
| Other audible and visual signals | Enhanced | Aria includes audible and visual signals for early lifting of device, inserting the wrong/used cassette, battery depleted |
| Cap lock | Enhanced | Aria has a safety feature which locks the cap onto the cassette to prevent removal of cap until the cassette is inserted in the device |
| Needle safety | Same | The needle sleeve extends when removing the device, locking in the extended position to prevent needlestick injury and contamination |
| Viewing window | Same | Aria has a large front-to-back window to view the drug in the syringe |
| Number of steps | Similar | Aria requires insertion and removal of the cassette (user steps are similar once the cassette is inserted). However, Aria benefits from inclusion of dwell time in injection completion notification |
| Size | Larger | Aria is more compact than other electronic devices and similar in length to disposables, particularly 2.25 mL devices |
Table 1: Comparing some use aspects of Aria to disposable single-use autoinjectors.
“The original vision for Aria – to come close to the size, form factor and user steps achieved by disposable single-use devices – was explored in Phillips-Medisize’s studies.”
The original vision for Aria – to come close to the size, form factor and user steps achieved by disposable single-use devices – was explored in Phillips-Medisize’s studies. The fact that Aria operates in a very similar way to two-step disposable devices, once the single-use cassette is inserted, was liked by users (Box 1). The extra step of inserting the cassette was not perceived to be a challenge by most users.
BOX 1: QUOTES FROM ARIA USER STUDIES
“Very easy, it’s spot on.” – On loading and using Aria.
“Yeah absolutely got the dose, the green light and check mark is a nice indicator, job done.” – On Aria green check mark light.
“You don’t have to count, it does it all for you.” – On the lack of a dwell time step.
“It was much better than expected, it was gentle to use and felt really good.” – On using Aria.
“Should be attractive even if it is a medical device, maybe even more so, want to be happy to use it.” – On Aria from a consumer perspective.

Figure 2: The drug-containing cassette has a large viewing window to enable users to see the progress of their injection.
Aria includes a large front-to-back viewing window to observe the drug (Figure 2). The device automatically turns on when the cassette is inserted, a feature incorporated into the design based on feedback from earlier user studies. This aligns the user experience more closely to that of a two-step disposable autoinjector. However, the device still retains a button to turn it on and off as many users expect this on an electronic device. Also, in line with two-step autoinjectors, there is no button to initiate the injection – activation is achieved by a moveable needle sleeve that also provides needle safety after the injection.
A key safety feature Phillips-Medisize added to the design to reduce unintended use and misuse was locking the cap onto the cassette until it is inserted into the device. This feature has been well received by pharmaceutical customers, who perceive advantages relating to drug wastage, needle contamination and reduction in drug degradation if a device is uncapped for a long period before use.

Figure 3: End of dose is signified by a distinct sound and green check mark.
As discussed prior, the ability for electronic reusable autoinjectors to provide enhanced audio and visual feedback is a key opportunity. Based on user feedback, Phillips-Medisize iterated the design to develop a clear audible and visual signalling sequence during delivery and a green check mark and end-of-dose sound to indicate dose completion (Figure 3), which was very positively received (Box 1, above).
Many injectable drugs require an additional dwell time after the plunger has stopped moving to ensure complete delivery and avoid a wet injection. As such, users are often instructed to hold their device against the skin after the dose completion sound for a prescribed period of time. With Aria, a continuation tone is provided during the whole injection time and then the end-of-dose signal and green check mark is provided after any dwell time. This simplifies and essentially removes a dwell time step from the user sequence compared with most spring-based devices (Box 1, above).
“In Phillips-Medisize’s latest user study, there were no early lifts from 26 uses. This is a very encouraging result, as early lifts are among the most, if not the most, common user errors.”
RESPONSE FROM USER STUDIES
In Phillips-Medisize’s user studies, these clear features have enabled very good injection success rates, low early lifting rates and very positive feedback from users, particularly regarding the dwell time inclusion. Users have noted how easy Aria is to use, citing the green check mark and audio signalling as particularly easy to follow. Notably, in Phillips-Medisize’s latest user study (with six experienced and seven injection-naïve participants), there were no early lifts from 26 uses. This is a very encouraging result, as early lifts are among the most, if not the most, common user errors.4
In this particular study, Phillips-Medisize provided a quick reference guide (QRG) and presented the participants with a scenario in which they were given the device and cassettes and told they needed to use it for a long period of time to treat a chronic disease. Without any training, but with access to the QRG, they were invited to perform a simulated injection. Phillips-Medisize captured their initial thoughts and perceptions on being told they had to use a device to self-inject, and then asked them again after having used the device.
At the beginning of the session, naïve participants were typically apprehensive about using the device. However, after trying out the device for a simulated injection, most users were reassured, with many commenting on how simple the device was to use. Several commented that the motor sound and robustness of the device made them feel assured and gave them a sense of comfort, in addition to a few noting that they thought that the motor-driven injection was gentler than spring-based disposable autoinjectors (Box 1, above). These observations have demonstrated the importance of more subtle aspects of device design to the overall user experience, beyond the ability to use the device correctly. A few participants stated that these factors could influence their choice of a device.
Considering the use of visual and audible signals to improve safety, the ability of Aria to halt the injection progress if there is an early lift and prevent any drug spray or waste was seen as positive, as were the warning sounds and lights to indicate that an early lift had occurred, or that a used cassette has been inadvertently inserted.
“Phillips-Medisize’s user studies showed that sustainability has a definite influence on device choice – users would choose a more sustainable device, such as Aria, provided it was comparable to other devices with respect to safety and precision.”
EXTERNAL INFLUENCES AND CONSUMERISM
Although sustainability is a global issue and a strong driver for pharma companies in selecting medical device technology,5 Phillips-Medisize had not anticipated it to factor into user opinion quite as strongly as it did in its studies. The Aria Smart Autoinjector has been designed with sustainability very much in mind, with a 50% reduction in waste compared with fully disposable autoinjectors being Phillips-Medisize’s target. It also anticipated to have a much lower environmental impact, with approximately 60% less CO2 emitted per injection, when the full product lifecycle is considered. Phillips-Medisize’s user studies showed that sustainability has a definite influence on device choice – users would choose a more sustainable device, such as Aria, provided it was comparable to other devices with respect to safety and precision.
Connectivity is an area of growing interest for pharma companies, as it can bring commercial benefits and contribute to patient engagement and patient centricity. As noted in a recent Deloitte article,6 in part accelerated by the covid-19 crisis limiting direct access to healthcare professionals, patients are taking more interest in managing their health and are seeking to communicate in new and different ways with their doctors. There are indicators that patients are changing their attitude to data privacy, showing more willingness to share data remotely with healthcare professionals.
In Phillips-Medisize’s studies, the company had positive feedback from users on this new paradigm, with the vast majority opting for a companion app with a device like Aria, if it could assist them with aspects such as reminders, dosing history and injection site rotation assistance. In the company’s most recent study, 10 out of 13 participants would opt for the companion app if they were using an Aria device. Evidence is emerging that connectivity can play a role in improving healthcare,7 and it is reassuring to see that patients are eager to support this.
The Deloitte article also points towards more consumer-based influences in healthcare, an aspect on which Phillips-Medisize gained insightful feedback during its user studies. As safety and ease of use of devices are addressed, there appears to be a shift from ensuring patients “are able” to use a device to them “wanting to use” a device. As with consumer products, personal choice becomes more important as patients make decisions aligned with their lifestyle.
As such, in crowded drug markets where differentiation around drug efficacy becomes harder, other factors, such as how a therapy fits into a user’s lifestyle, come into play. In Phillips-Medisize’s user studies, participants emphasised the importance of the appearance of the Aria device, liking its “modern” look and feel. Equally, many saw the device’s colour and design having a role to play not as a matter of choice, but how it may make them feel – many felt strongly that devices could be in attractive colours rather than the more standard medical colours such as white and blue (Box 1, above). Also, because these devices are intended for use outside of a hospital setting, they are more a “part of life” and need to be attractive and make a user feel “happy to use” them.
CONCLUSION
Ease of use for a self-injection device remains a leading driver in its uptake. Although this can be achieved by both disposable and reusable electronic devices, such as Aria, it is clear from Phillips-Medisize’s research, and in results published elsewhere, that improvements can be made by the latter.
Key benefits include:
- Clearer and more persistent audio and visual feedback of both dose progress and completion
- Elimination of the need to manually count out the dwell time prior to lifting the device
- The ability to pause or stop injection if the device is lifted early from the injection site, meaning there is no drug spray or waste, or drug on skin
- The ability to lock the cap in place before injection to reduce the risk of premature cap removal and enabling a timeout or warning if the injection is not completed soon after the cap is removed and the sterile barrier broken
- Detection of early lifting, allowing user feedback to enable course correction.
“Considering broader issues, including sustainability and consumer preferences such as feel, look and style, there is a strong argument in favour of reusable electronic autoinjectors over their conventional mechanical counterparts.”
Considering broader issues, including sustainability and consumer preferences such as feel, look and style, there is a strong argument in favour of reusable electronic autoinjectors over their conventional mechanical counterparts. Although evaluating the merits of these points requires further research and analysis, with over 75% of participants from Phillips-Medisize’s user studies preferring Aria over a single-use disposable, there is already a strong case in their favour from a user point of view.
Factoring in better sustainability, which is valued by users and pharma companies alike, as well as the flexibility to adapt the design to meet the needs of broad drug portfolios and the economies of scale through development and industrialisation of the product as a platform, Phillips-Medisize is confident that there is an exciting future for reusable electronic products such as Aria. Over the coming few years, Phillips-Medisize will continue to develop and test the design, firstly in further user studies, then in clinical trials with customers, which are planned for 2022. Phillips-Medisize anticipates that the first finished combination products utilising the Aria Smart Autoinjector platform will be introduced into market by its customers towards the end of 2023, pending approval by the FDA and similar regulators in other jurisdictions.
REFERENCES
- Weinhold T et al, “Improving the Safety Of Disposable Auto-Injection Devices: A Systematic Review of Use Errors”. AAPS Open, Oct 2018, Vol 4, Article 7.
- Calawa B, “Staying Dry: How to Limit Wet Injections in Auto-Injector Design”. Med Device Online, Nov 2015.
- Collier DH et al, “A Novel Electromechanical Autoinjector, Autotouch™, for Self-Injection of Etanercept: Real-World Use and Benefits”. Postgrad Med, Jan 2017, Vol 129(1) pp 118–125.
- Potera C, “Misuse of Autoinjectors and Inhalers”. Am J Nurs, Mar 2015, Vol 115(3), p 17.
- Carpenter G, “Trend Report – Sustainability in Pharma”. CPhI Online, Jun 2021.
- Betts D, Korenda L, Giuliani S, “Are Consumers Already Living the Future Of Health?”. Deloitte Insights, Aug 2020.
- Bittner B et al, “Connected Drug Delivery Devices to Complement Drug Treatments: Potential to Facilitate Disease Management in Home Setting”. Med Devices (Auckl), Mar 2019, Vol 12, pp 101–127.

